Dendritic cells are indispensable for ovulation
Adva Cohen Fredarow, Tal Raz and Naama Meterani
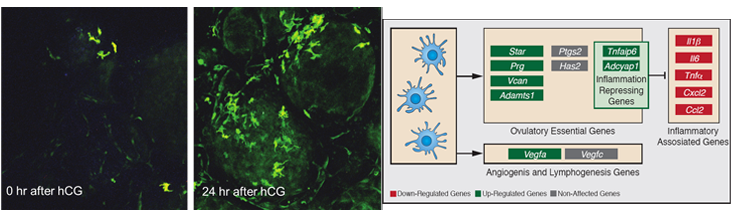
The suggested analogy between ovulation and an inflammatory response takes into account the fact that during ovulation follicles become hyperemic, produce prostaglandins and synthesize a hyaluronan-rich extracellular matrix. This idea goes along with the preovulatory LH-induced upregulation of inflammatory-associated genes, increase in vascular permeability and invasion of immune cells into the ovarian tissue. We hypothesized that these immune cells, specifically dendritic cells (DCs), play a role in ovulation and corpus luteum formation.
To examine modifications in DCs abundance and determine their localization we used transgenic mice, in which the expression of the DCs marker, CD11c is conjugated to YFP and conditional ablation under the control of the CD11c promoter sequence. We found that CD11c positive, F4/80 negative cells, apparently representing DCs, reside in the ovary prior to ovulation and massively accumulate in the newly formed corpus luteum. Our study further revealed that CD11c positive cells are absolutely essential for the release of the ovum from the ovarian follicle, as well as for the formation of a functional corpus luteum.
We also demonstrated that these effects of CD11c positive cells are mediated by upregulation of ovulation-essential genes and stimulation of lymphangiogenesis. Unexpectedly, we detected a
remarkable anti-inflammatory capacity of the CD11c positive cells, which seemingly serves to restrict the ovulatory-associated inflammatory response. Our results provide, for the first time, strong evidence for the involvement of the ovarian DCs in controlling the ovulatory response.