This research line addresses a long-standing problem in the field of nerve regeneration: how does the cell body receive information about an injury from the distant lesion site in the axon. We started by examining involvement of nuclear import factors, showing that importin β1 mRNA is located in axons and translated locally upon injury.
The importins complex is transported retrogradely via an interaction with dynein, and blocking the process inhibits regeneration. Thus, regeneration is triggered by signals from the injury site that are transported by an importin/dynein complex (Hanz et al, 2003). We then used differential proteomics to search for signaling components of the complex, showing that soluble truncated forms of vimentin transport phosphorylated ERKs (pERK) in injured rat or mouse sciatic nerve, by linking pERK to the retrograde motor dynein via an association with importin β1.
Strikingly, vimentin protects the pERK from phosphatases en route, thus establishing a novel mechanism for long distance transport of an activated kinase (Perlson et al., 2005). In more recent work we demonstrated that Ran GTPase and its associated effectors regulate the formation of importin signaling complexes in injured axons, by providing a locally regulated ‘safety catch’ that prevents inappropriate importin association (Yudin et al., 2008). A comprehensive characterization of signaling to transcription networks after axonal injury revealed transcription factors and other regulators of the regeneration response that are trafficked by this mechanism (Michaelevski et al., 2010; Ben-Yaakov et al, 2012). Subcellular knockout of axonal importin β1 by specific targeting of a 3’UTR sequence confirmed the central role of local axonal synthesis of importins in retrograde injury signaling (Perry et al., 2012). These findings established novel roles for importins and their regulators in cytoplasmic signaling and transport, with broad implications for integration of cytoplasmic and nuclear transport mechanisms in both normal and injured cells.
In more recent work we have focused on how local translation is regulated in the axon. A number of RNA Binding Proteins (RBPs) traffic importin β1 and other key mRNAs to axons, most prominently an RBP called nucleolin (Perry et al., 2016). Nucleolin also transports mTOR mRNA to axons, and localized regulation of mTOR translation is critical for regulation of local protein synthesis after axon injury (Terenzio et al., 2018). Our current research priorities in this line are understanding the mechanisms that control subcellular localization of nucleloin, determining the roles of additional axonal RBPs, and elucidating additional regulatory mechanisms that control local translation in axons.
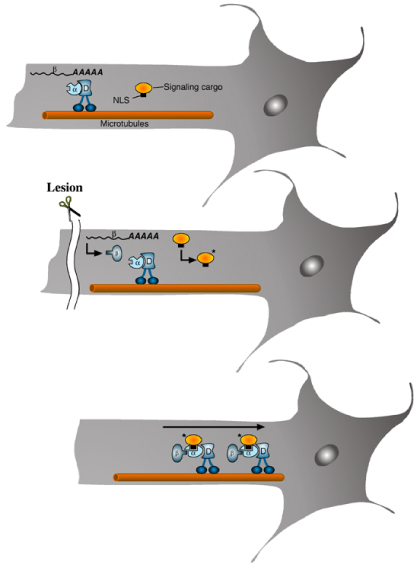
Importin-alpha protein is constitutively associated with the retrograde motor dynein (D) in axons, whereas importin-beta is normally present only as mRNA (upper panel). Upon lesion, local translation of importin-beta to protein induces formation of the importin heterodimer, thus creating a high affinity NLS-binding site associated with dynein. Concomitant modification of NLS-bearing signaling proteins in axoplasm creates a signaling cargo that binds to the complex, thus accessing the retrograde transport pathway (middle and lower panels).