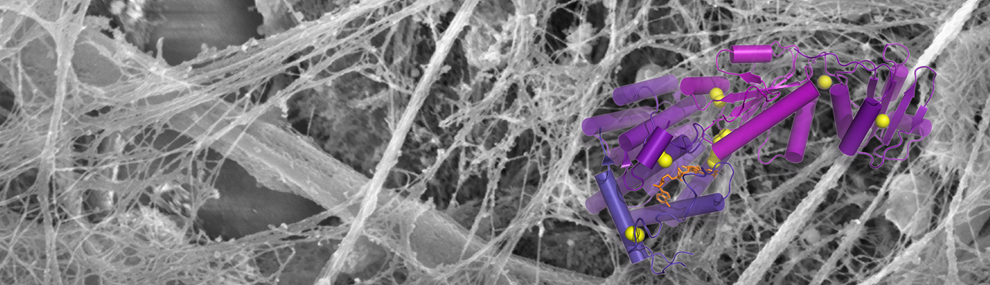
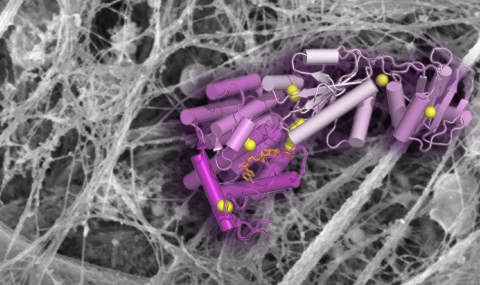
Mucins are huge glycoproteins that coat the inner surfaces of the lungs, intestines and other organs to protect them from pathogens and mechanical damage. Using electron microscopy X-ray crystallography, and biochemical techniques, we have begun to reveal the mechanisms by which mucins form disulfide-linked polymers. In particular, we have shown how low pH corresponding to the Golgi apparatus promotes supramolecular assembly of mucin polymerization intermediates. We are also beginning to understand how specific molecular features of mucins lead to their structural and functional diversity.
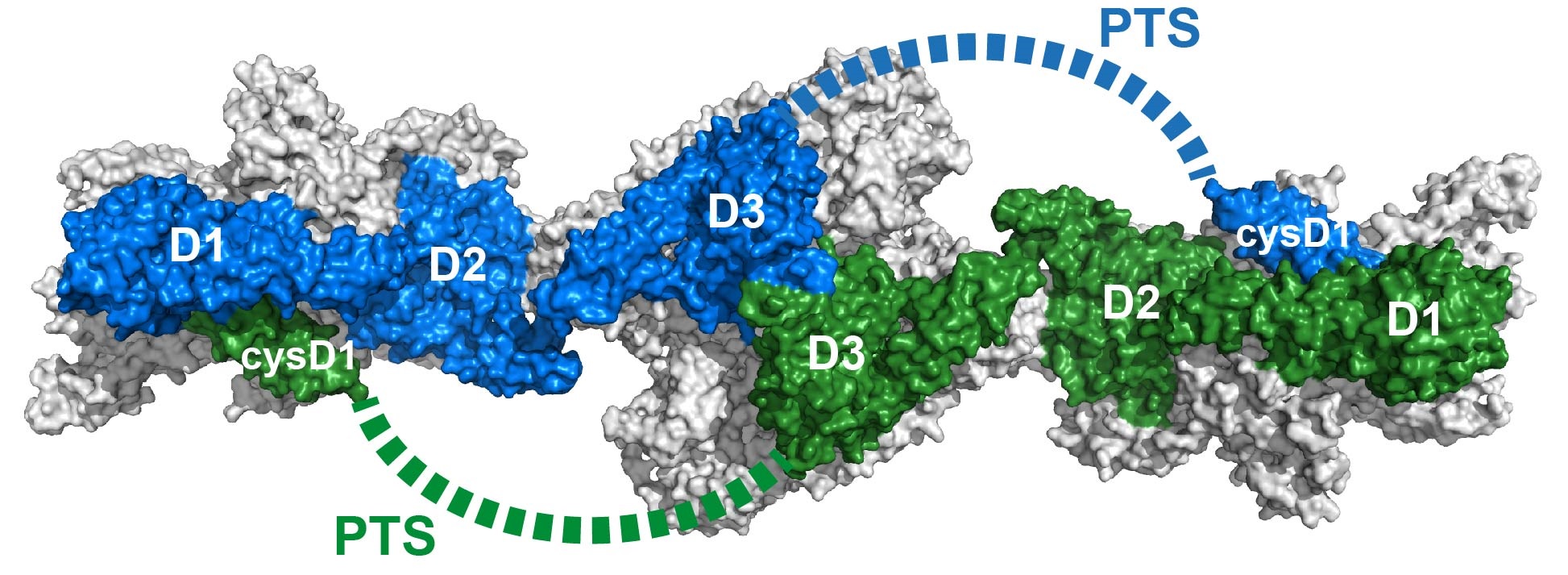
[background-color:white]