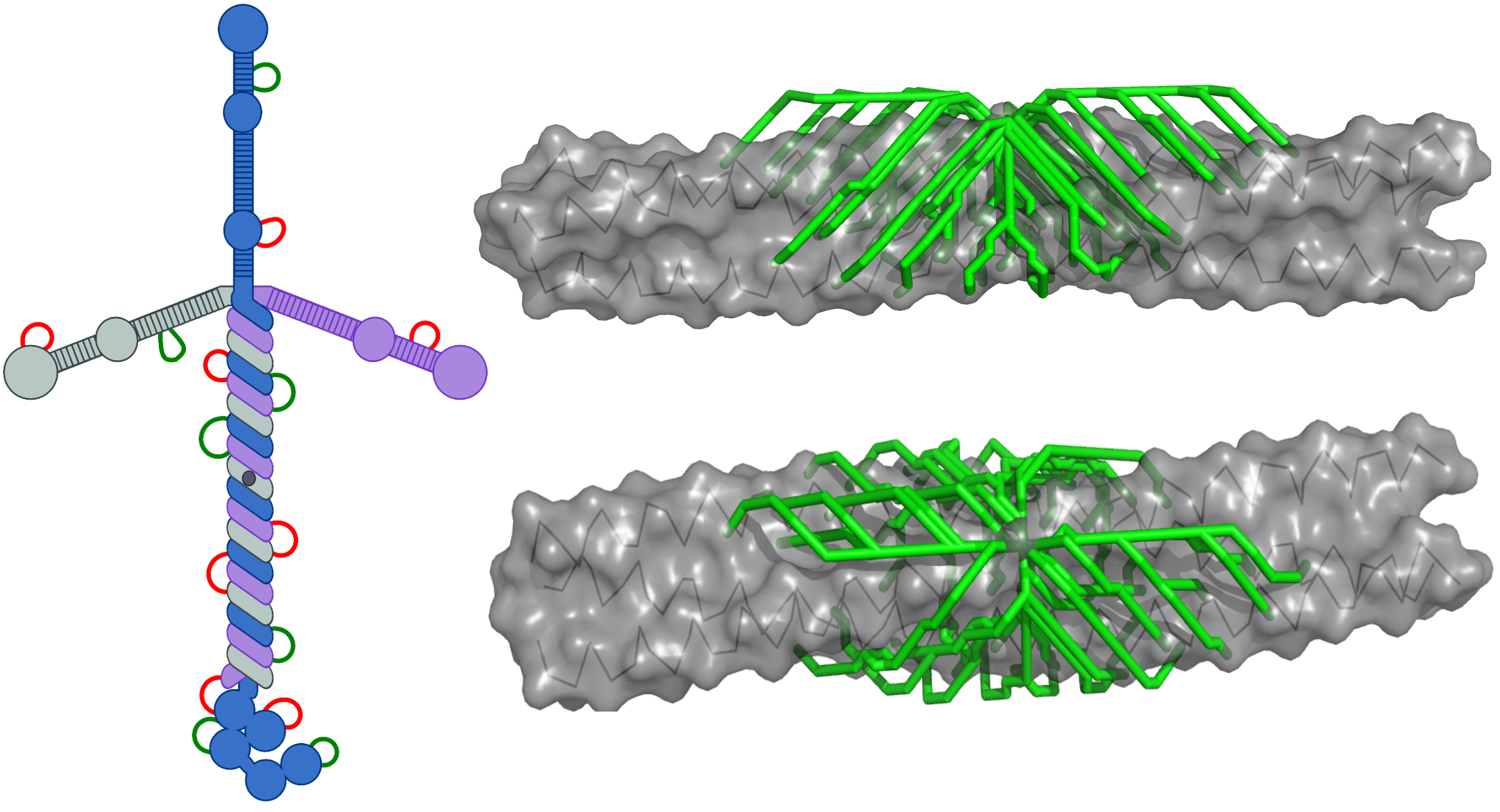
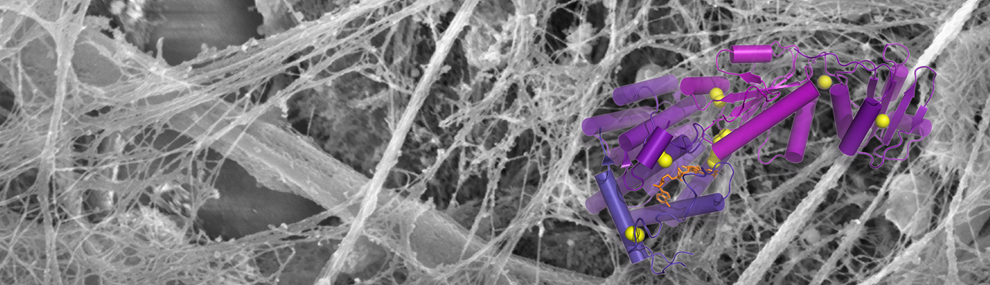
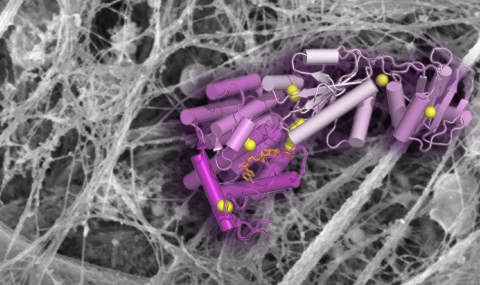
We have used cross-linking and mass spectrometry to elucidate the quaternary structure of laminin, an important component of the extracellular matrix. Laminin is a heterotrimeric protein with a total molecular mass of about 800 kD and is held together by a three-stranded coiled coil spanning about 750 Å (~550 amino acid residues). We discovered that the order of the three subunits in the trimer is opposite to that previously proposed. The correct subunit order is a fundamental piece of information needed to properly consider mechanisms of laminin assembly. We are currently working on improving experimental methodologies and data analysis to extend this work to more complex, assembled extracellular networks. Our aim is to reveal novel interactions between different extracellular matrix proteins and new modes of self-assembly within matrix fibers.