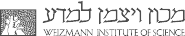More than 80% of all eukaryotic transcription factors contain disordered regions and a significant fraction of them are longer than 50 amino acids. These proteins are involved in a wealth of regulatory cellular processes such as cell-cycle control, chromatin remodelling, or control of homeostasis. It is therefore important to understand the functional advantages of these disordered regions compared to ordered domains.
We address this problem by an in-depth investigation of their polymeric properties. Ultra-fast single-molecule correlation methods with picosecond time resolution are used in combination with concepts from polymer physics to understand the relation between conformation, dynamics, and function.
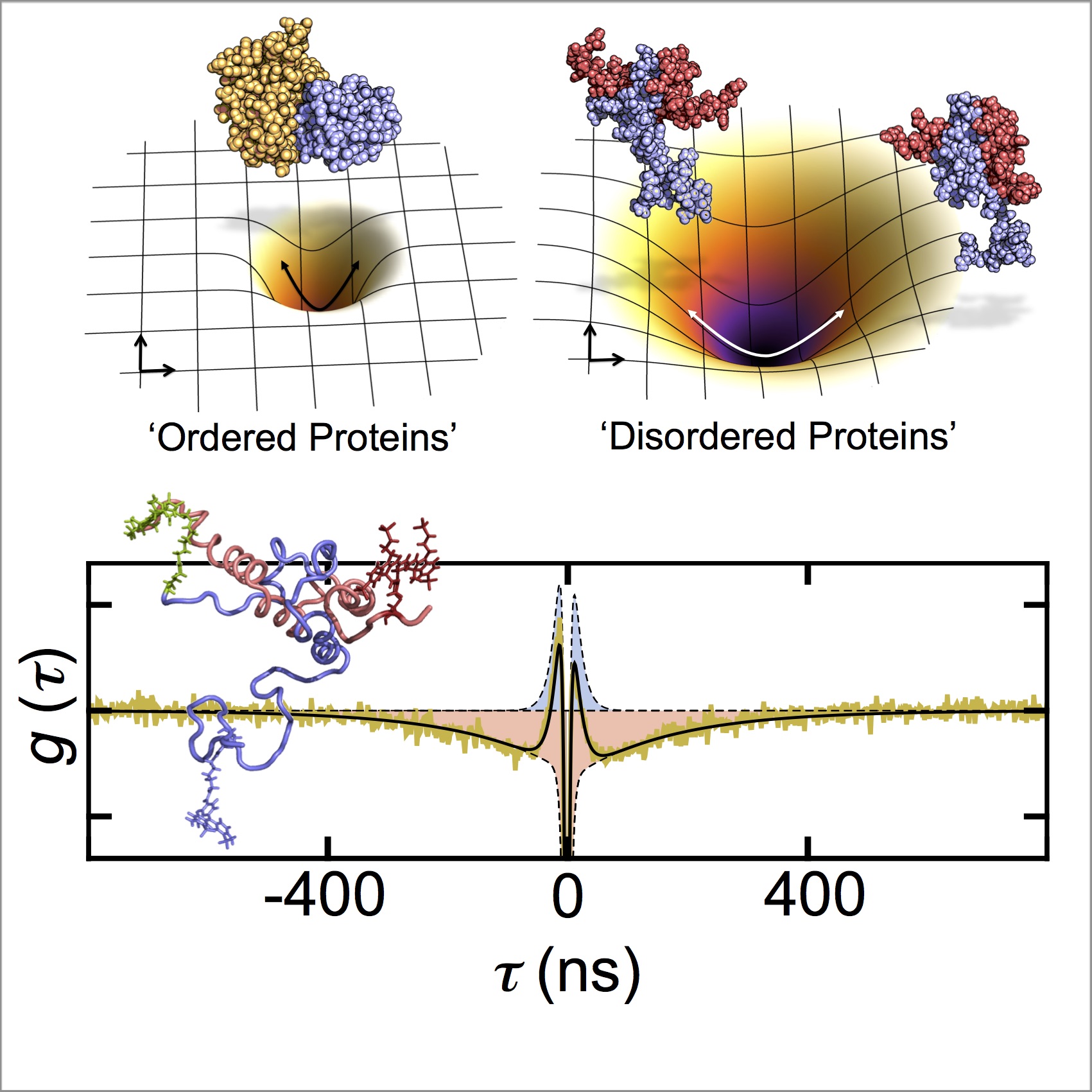
We use ultra-fast photon correlations to directly monitor the dynamics of flexible disordered proteins and their complexes.