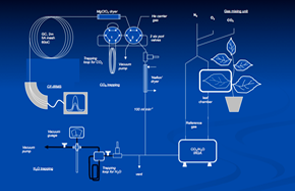
Our stable isotope lab is equipped with two Isotope Ratio Mass Spectrometers (IRMS) with the necessary peripheral instrumentations to provide capabilities for isotopic analysis of atmospheric CO2, water and water vapor, and organic materials. These analyses focus on 13C and 18O in these materials and allow both on-line and off line analytical approaches. These systems include direct gas analysis for analysis of CO2, O2 and N2 in air samples, as well as water pyrolysis to CO for 18O analysis of water and water vapor samples, and combustion CHN analyzer for the organic samples form plant and soil samples (both 13C and 18O).
A lab technician runs our stable isotope lab but direct access and operation for graduate students as needed is allowed for their research, including the on-line connection of gas-exchange and ‘micro-cosmos’ experimental system.

Dan Yakir, Shani Rohatyn, Yakir Preisler,
Efrat Ramati, Fedor Tatarinov
Below: Efrat Schwartz
In the past, our stable isotope lab developed the capability to run a network of canopy air sampling for “Keeling plots” analysis across Europe that allows shipment of sets of flaks that can be adapted to standard Flux Tower sites in the Fluxnet community and returned to our lab for automatic isotopic analysis.
Our stable isotope analyses usually show precision for the water analysis is ±0.1‰, for CO2 from the on-line discrimination measurements an external precision of ±0.05‰ for δ13C and ±0.1‰ for δ18O. δ18O values of small water samples are determined by pyrolysis on a carbon catalyst and "on-line" analysis of the product CO with a precision of ±0.2‰. Standards for isotopic analysis are routinely obtained from IAEA (Vienna). CO2 concentrations and isotopic values of reference gases will be based on routine inter-comparison the NOAA-CMDL global network in Boulder.
Figures: “On-line” microcosms” experiments carried out in the stable isotope lab to develop our understanding of the plant influence over the isotopic signals observed in the atmosphere.
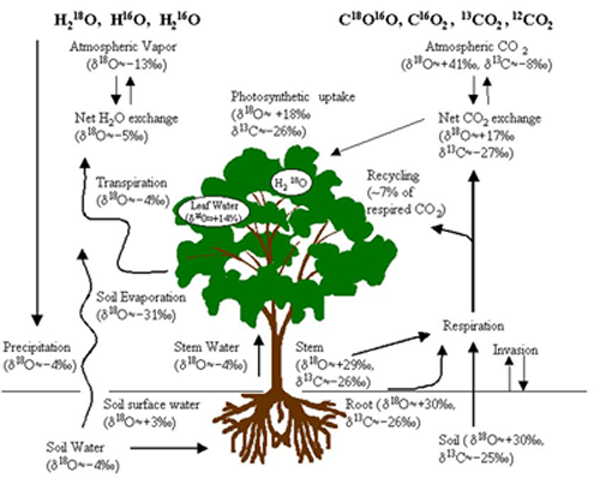
Major components for the use of stable isotopes in ecosystem gas exchange: The values are rough approximations and can vary greatly with geographical location and environmental conditions (given for demonstration purposes only and based mostly on data from Israel). The main advantages of the isotopic approach lie in the unique labeling of flux compnents: Photosynthesis (depleted uptake) tends to enrich the atmosphere, while respiration (depleted release) tends to deplete the atmostphere in 18O and 13C; leaf transpiration and soil evaoration are isotopically very different fluxes; root and soil respiration can have distinct 13C labeling. (Values are on the SMOW and PDB scales for δ18O and δ13C values respectively).