Redox regulation of O-linked carbohydrate addition in mucosal tissues.
Area:
Chemistry
Life Sciences
Sunday, September 5, 2021
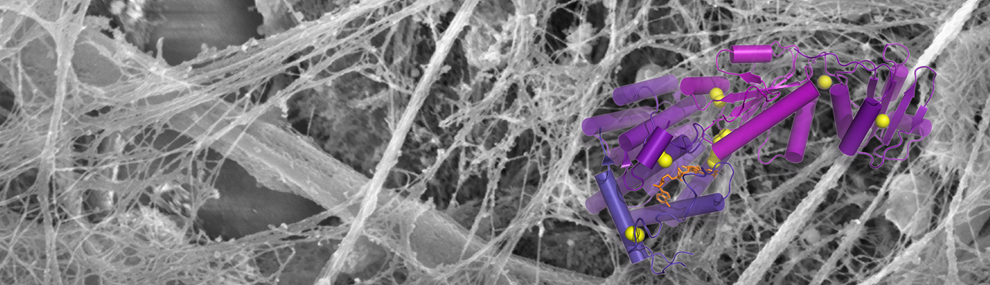
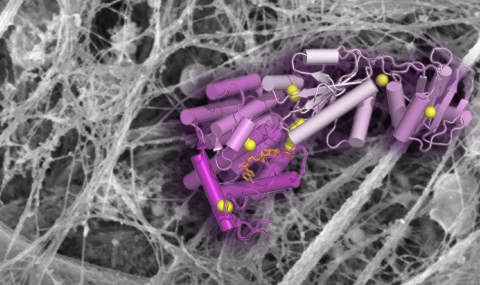
O-linked carbohydrates play an important part in cell adhesion, combating viral infection, and many other important physiological processes. Our lab has recently discovered that a catalyst of disulfide bond formation regulates a set of enzymes in the Golgi apparatus that control O-glycosylation in musocal tissues. A Ph.D. project following up this finding can develop in the direction of structural biology, biochemistry, cell biology, and in vivo studies, according to the student's interests. No prior experience necessary, we will teach you everything you need to know!