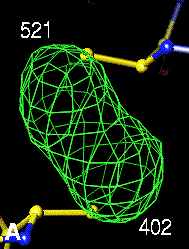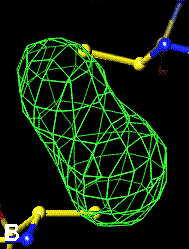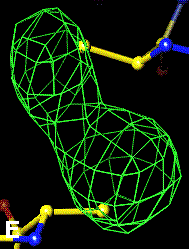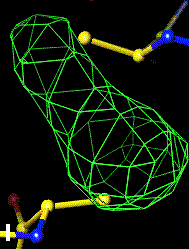
Below are four animations, one in each panel. Each frame is a complete data-set collected in about 3.5 minutes, each data-set is a 15 minutes time point. In the refinement, the Cys were replaced with Ala to minimize model bias.
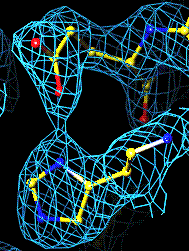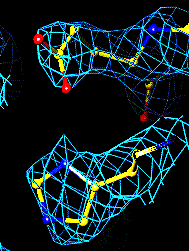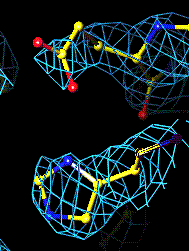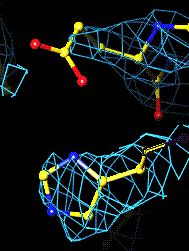
The top left panel is the 3Fo-2Fc map and model of the 254-265 disulfide bridge, and the top right panel is the Fo-Fc map of the same S-S bond.
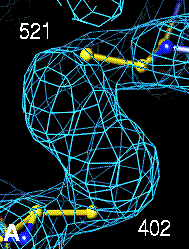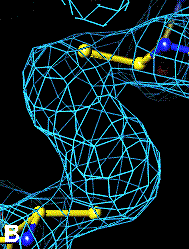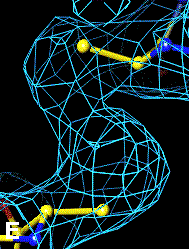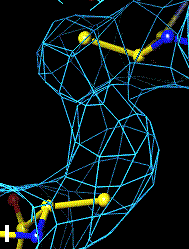
The bottom left and right panels are for the 402-521 bridge.
|
3Fo-2Fc map of 254-265 S-S bond |
Fo-Fc map of 254-265 S-S bond |
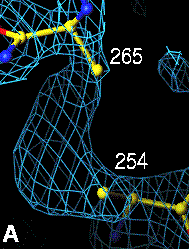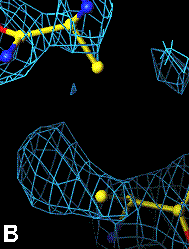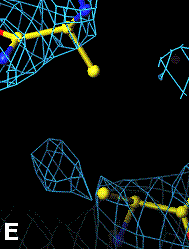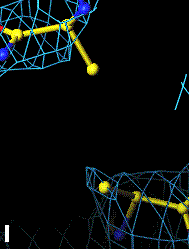 |
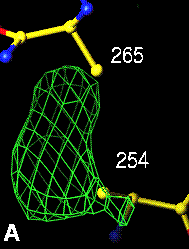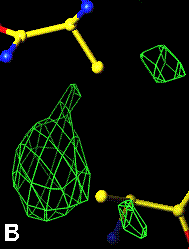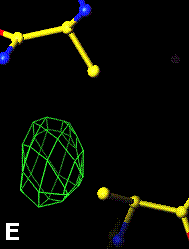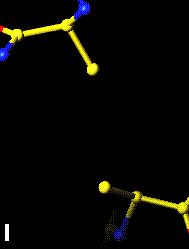 |
|
3Fo-2Fc map of 402-521 S-S bond |
Fo-Fc map of 402-521 S-S bond |
 |
 |
|
The Active Site 3Fo-2Fc map of Glu 327 and His 440 |
 |
 PNAS, Vol. 97, Issue 2, 623-628, January 18, 2000
PNAS, Vol. 97, Issue 2, 623-628, January 18, 2000
Specific chemical and structural damage to proteins produced by synchrotron radiation
Departments of * Crystal and Structural Chemistry and ¶ NMR Spectroscopy, Bijvoet Center for Biomolecular Research, Utrecht University, Utrecht, NL-3584 CH, The Netherlands; b European Molecular Biology Laboratory Outstation, Grenoble, 38042 France; Departments of § Structural Biology and c Neurobiology, Weizmann Institute of Science, Rehovot, 76100 Israel; and d Biology Department, Brookhaven National Laboratory, Upton, NY 11973
Communicated by F. William Studier, Brookhaven National Laboratory, Upton, NY, November 16, 1999 (received for review October 6, 1999)
Radiation damage is an inherent problem in x-ray crystallography. It usually is presumed to be nonspecific and manifested as a gradual decay in the overall quality of data obtained for a given crystal as data collection proceeds. Based on third-generation synchrotron x-ray data, collected at cryogenic temperatures, we show for the enzymes Torpedo californica acetylcholinesterase and hen egg white lysozyme that synchrotron radiation also can cause highly specific damage. Disulfide bridges break, and carboxyl groups of acidic residues lose their definition. Highly exposed carboxyls, and those in the active site of both enzymes, appear particularly susceptible. The catalytic triad residue, His-440, in acetylcholinesterase, also appears to be much more sensitive to radiation damage than other histidine residues. Our findings have direct practical implications for routine x-ray data collection at high-energy synchrotron sources. Furthermore, they provide a direct approach for studying the radiation chemistry of proteins and nucleic acids at a detailed, structural level and also may yield information concerning putative "weak links" in a given biological macromolecule, which may be of structural and functional significance.


