For more than thirty years, I have had the rare opportunity to be able to work closely with Prof. Israel Silman, of the Neurobiology Dept, on studying proteins involed in neural transmission via cholinergic mechanisms, Our work has been focused on studies of the synaptic enzyme, acetylcholinesterase (AChE), whose principal role is termination of synaptic transmission at cholinergic synapses by rapid hydrolysis of the neurotransmitter, acetylcholine. For over 50 years, AChE has been the subject of intense interest for enzymologists, pharmacologists and toxicologists, due to its extremely high catalytic activity, a requirement for its biological activity, and since it is the target of the first generation of anti-Alzheimer (AD) drugs and of potent nerve agents and insecticides.
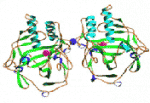
From the onset, our joint objective was determination of the 3D structure of AChE so as to understand its mechanism of action and permit structure-based drug design. Although attempts started in the ’60s, no crystals suitable for 3D structure determination were obtained prior to our pioneering work. We developed a novel, mild approach to solubilization of membrane-bound AChE from Torpedo californica electric organ (TcAChE), followed by its crystallization, and then by solution of its 3D structure. Our publication of the structure, as a full paper in Science, has been cited over 2,450 times, and many ensuing papers hundreds of times. We subsequently solved and published two additional important AChE structures, those of human and Drosophila AChE. Concomitantly, we solved the structures of more than 40 complexes and conjugates of AChE with a broad repertoire of drugs, toxins and other ligands. These included essentially all the 1st generation anti-AD drugs, several of the major nerve agents, insecticides, transition-state analogs and a snake venom toxin. These structural data provide an essential reservoir of information for generation of novel lead drugs. We have published over 170 joint papers.