While advances in medicine have increased the life expectancy of the overall population, the number of people affected by age related pathologies has also increased. The challenge facing physicians and scientists, then, is not just to help people live longer, but to be healthy and functional during their extended lifetimes. One of the most common afflictions of old age is senility, or dementia, which is the inexorable decline of cognitive ability, beginning with memory loss, and ending with complete helplessness. The most common cause of dementia is Alzheimer's disease (AD), affecting about 10% of the population over 65, 25% of the people over 75, and up to 45% of the people over 80. It is estimated that there are currently 4.5 million people suffering from Alzheimer's disease in the US alone.
While no curative treatment has been found for AD, palliative drugs are available which help counteract memory loss. According to the cholinergic hypothesis, memory loss in Alzheimer¹s disease in due to decreased levels of the neurotransmitter acetylcholine (ACh) in cholinergic synapses. In normally functioning synapses, the enzyme acetylcholinesterase (AChE) is responsible for rapid breakdown of ACh to allow repeated signal transmission. Inhibition of AChE in patients sufferening with AD should allow the lowered levels of ACh in the synapses a chance to induce a signal in the downstream nerve.
We have solved the 3D structure of AChE, as well as several compounds complexed with AChE. A number of these compounds either have been, or are currently used as drugs in treating AD, while others are potential drugs.

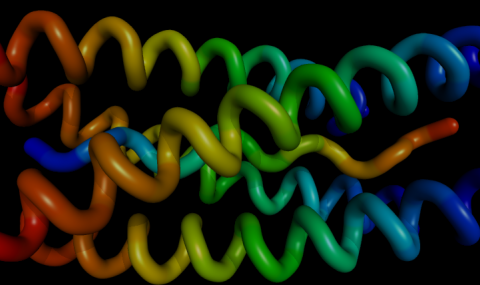
Ribbon diagram of the crystal structure of the HupA-TcAChE complex. Green arrows represent β strands, and brown coils, α-helices. The side chains of the catalytic triad and of key aromatic residues in the active-site gorge are indicated as purple stick figures. The HupA structure, which was experimentally determined in this complex, is represented as a space-filling model, with carbons shown in yellow, oxygens in red and the nitrogen in blue.
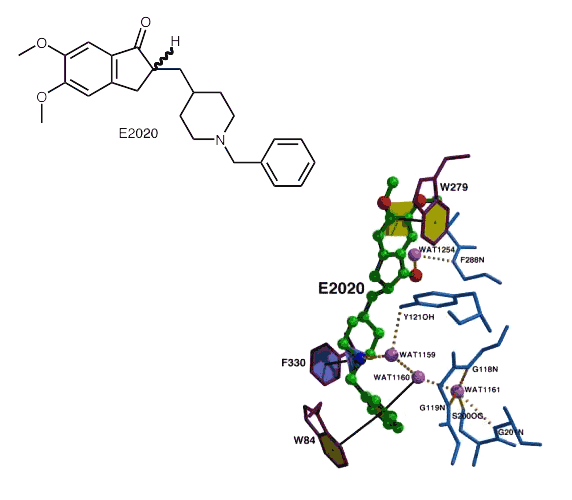
Binding of E2020 to TcAChE. E2020 is displayed as a ball-and-stick model. Water molecules are represented as light purple balls; Œstandard H-bonds as heavy dashed lines; aromatic H-bonds, π-cation and stacking interactions as black lines. Note the multiple water-mediated contacts of E2020 atoms with the protein catalytic.
Kryger et al. (1997) Structure 7, p297.

This compound is marketed as Exelon, and bonds covalently to the enzyme. The leaving group also remains bound to the enzyme. The active site histidine moves significantly from its native position, disrupting the catalytic triad.

Since Huperzine A is a natural product, and so not patentable, and its synthesis is difficult, derivatives are sought to make their production more economical. Huprine X is a hybrid between Huperzine A and Tacrine, a compound once used as an AD drug, but discontinued because of its hepato-toxcicity.
Ribbon diagram of the crystal structure of the HupA-TcAChE complex. Green arrows represent β strands, and brown coils, α-helices. The side chains of the catalytic triad and of key aromatic residues in the active-site gorge are indicated as purple stick figures. The HupA structure, which was experimentally determined in this complex, is represented as a space-filling model, with carbons shown in yellow, oxygens in red and the nitrogen in blue.
Greenblatt et al. (1999) FEBS Letters 463, p321.