Fundamental catalysis
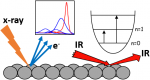
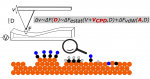
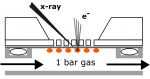
We study the physical and chemical phenomena on model catalyst surfaces. Our studies are relevant to the currently prevalent grands challanges in energy and environment, and potential roadmaps such as the hydrogen economy, methanol economy, oil economy, and carbon dioxide utilisation.