cells throughout our body constantly interact with their microenvironment. While biochemical communication has been extensively studied for a long time, the importance of mechanical interactions (i.e. cells' ability to apply, sense and respond to forces) has been recognized only recently. Precise mechanical conditions, from the subcellular level and up to the organ scale, are critical for tissue development, function, remodeling and healing. Nevertheless, understanding the precise nature of the mechanisms and processes underlying the response of living cells to mechanical cues - cellular mechanosensitivity - remains largely a basic open problem.
Recently, we experimentally and theoretically studied one striking manifestation of cellular mechanosensitivity: cell reorientation in response to cyclic stretching of the underlying substrate. In the experiments, we focused on the response of cells to cyclic stretching which mimics vital physiological conditions (e.g. heart beating, pulsating blood vessels and breathing), where adherent cells starting from naturally random orientations - reorient to a well-defined and uniform angle which depends on the applied stretching. This outstanding process reveals high cellular sensitivity and accuracy in response to external forces. We then developed a new theory, which takes into account both the passive 2D elastic anisotropic response of the cells to substrate deformation and the active remodeling of their actin cytoskeleton and focal adhesions (FAs), highlighting a fascinating interplay between structure, elasticity and molecular kinetics in the reorientation process. The theory is in excellent quantitative agreement with all of the extensive experimental data, predicting the complete temporal reorientation dynamics. For more details see the paper and figure below.
Other ongoing projects focus on the active nature of actomyosin contractility, i.e. the physics of myosin II motors that actively contract the actin network in cells, and on actin severing, a process that is essential for cellular processes such as division, growth and motility.
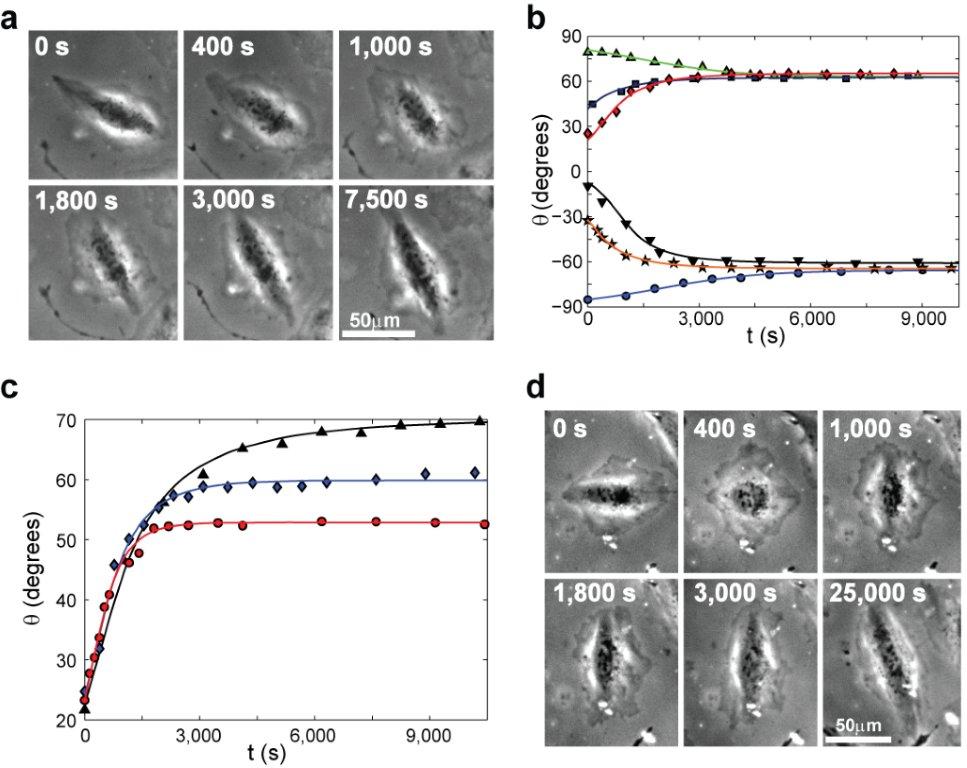
Phase contrast snapshots tracking single cell reorientation dynamics under cyclic stretching. The solid curves in panels b & d are theoretical predictions, which exhibit excellent agreement with the reorientation dynamics of single cells.
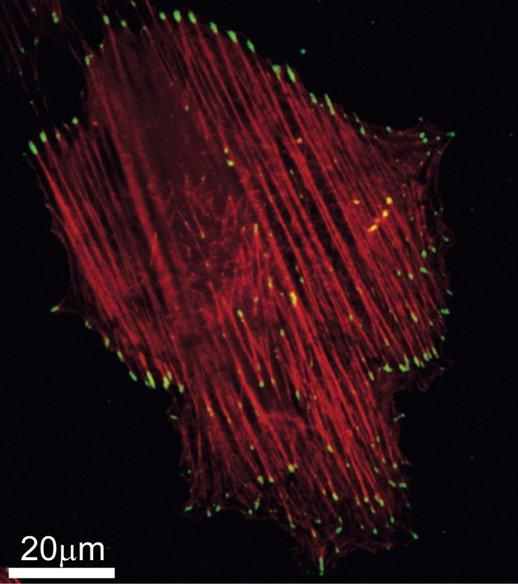
Stress Fibers (red), mainly made of actin, and Focal Adhesions (yellow), molecular complexes which couple to the SFs ends and adhere to the underlying substrate, in a single cell after 6 hours of cyclic stretching.
Selected publications
-
Tissue-level mechanosensitivity: Predicting and controlling the orientation of 3D vascular networks
S. Landau, A. Moriel, A. Livne, M-H. Zheng, E. Bouchbinder, S. Levenberg
Nano Lett., Just Accepted Manuscript (2018) -
Cell reorientation under cyclic stretching
A. Livne, E. Bouchbinder, B. Geiger
Nature Comm. 5, 3938 (2014). arxiv 1412.0369


