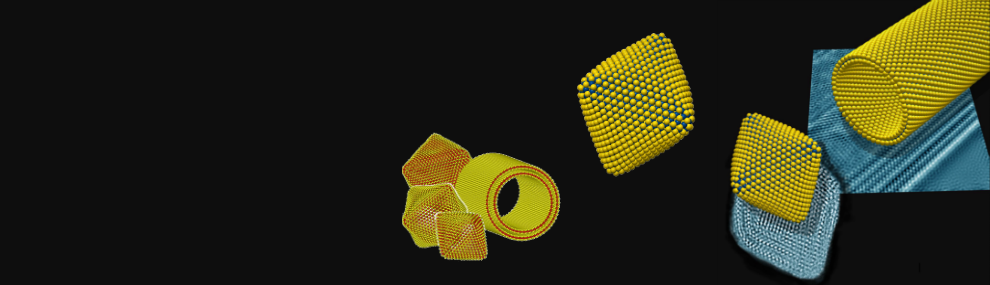
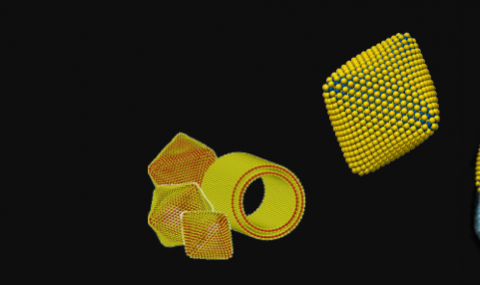
One of the most challenging questions concerns the structure and properties of the smallest IF nanoparticles. As early as 1993 (No. 97), we have proposed that rectangles and triangles may be formed in the apex of the IF nanoparticle. Decorating the MoS2 layer with six such rectangles, symmetrically, we proposed that hollow nanooctahedra could be formed (No. 1 in the list of “invited review articles”). This idea was verified by Heben et. al., (Nature 397, 114 (1999)), who used laser to ablate MoS2 and obtained hollow MoS2 nanooctahedra. They termed these nanostructures as the “true inorganic fullerenes”. Combining ab-initio calculations by Prof. G. Seifert (Fig. 8) with TEM analysis the detailed structure of the MoS2 nanooctahedra was studied (No. 217, 220 and 233- see also Fig. 9). Using solar-ablation (No 255) Prof. J.M. Gordon, Prof. D. Feuerman (Ben-Gurion University) jointly with our group and that of Prof. G. Seifert (TU Dresden) the window of stability fort the MoS2 nanooctahedra (the “true inorganic fullerenes”) was determined to be between 103 to 105 atoms- see Fig. 10. The nanooctahedra are calculated to possess a metallic character and could be useful catalysts.
|
|
Fig. 8. The dependence of the energy per atom Et/N on the total number of atoms N for various MoS2 nanostructures: triangular nanoplatelets (blue curve), octahedral fullerenes with k shells (red curves), and spherical fullerene-like nanoparticles (green curves) with k shells (from Ref. 220) |
| Fig. 9. DFTB optimized structure of a hollow Mo576S1140 (left) and a Mo576S1140 nanooctahedra after an MD experiment at 300 K (right). Courtesy of Prof. G. Seifert, Technical University Dresden, Germany (from Ref. 220). |
|
 |
Fig. 10. TEM image of a hollow MoS2 nanoparticle with inner core made of 8-layers with nanooctahedron structure. The larger layers beyond the nanooctahedron are sheathed more uniformly, giving rise to a quasi-spherical or polyhedral shape IF nanostructure. Superimposed on the TEM image is a schematic rendering of the nanoparticle (courtesy of Dr. A. Enyashin, Solid State Chemistry Institute, Ekaterinburg, Russia). See reference No. 255. |