
Caco-2 cells labeled for tight junction molecule cingulin (green), actin (red), vinculin (pink) and DNA (blue).

Epithelial cells growing on a patterned adhesive surface with the shape of the Weizmann Institute tree.

Desmosomes in mouse tongue epithelium (by transmission electron microscope).

Porcine aortic endothelial cell, double-labeled for actin (green) and phospho-tyrosine (red).

“Molecular composition map” of focal adhesions and stress fibers.

Myeloma cancer cell responding to shear flow (by scanning electron microscope).
You are here
Scientific Activities ››Cell biology of osteoclasts
Bone homeostasis and remodeling depend primarily on the coordinated action of two cell types – osteoclasts that degrade bone, and osteoblasts that deposit new bone. Lack of balance between the activities of these two cell populations leads to serious bone disorders; namely, osteoporosis, a rather common situation in which bone mass and density is decreased, and osteopetrosis in which bone density becomes pathologically high. The mechanism underlying physiological and pathological bone remodeling raises some interesting questions such as how do osteoclasts and osteoblasts “decide” which areas of the bone should be remodeled? Do they “sense” the surface, and if so – how do they do it? Are the adhesions of these cells to the bone tissue involved in the “surface sensing process”? How is bone resorption regulated, and what is the involvement of the cytoskeleton in this process? Studies in the lab that address these and related questions are conducted in collaboration with Prof. Lia Addadi (Department of Structural Biology, Faculty of Chemistry at the Weizmann Institute).
Our lab has characterized the primary building blocks of the “resorptive apparatus”, namely, podosomes, that consist of an actin core bundle that extends from the ventral (“bottom”) plasma membrane toward the cytoplasm. This actin-based core is surrounded by an adhesion ring containing a variety of adhesome components (see Figure 1 and the section on the integrin adhesome network).
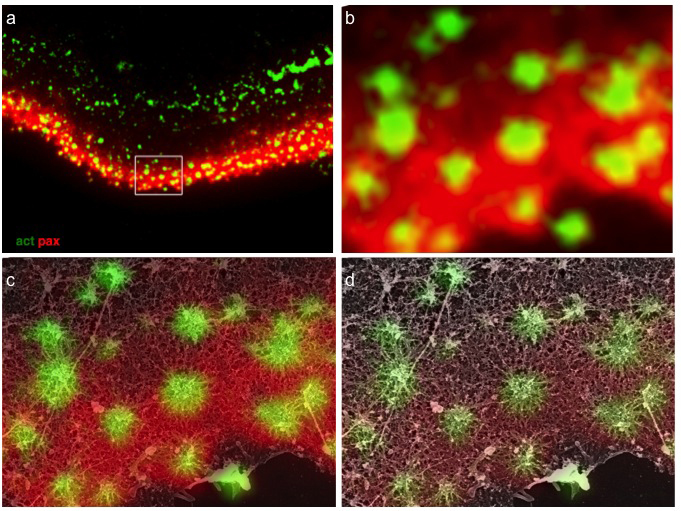
Figure 1
Structural relations between podosome ring and core domains. (a) Osteoclast ventral membranes were labeled for paxillin and actin, and simultaneously prepared for high-resolution scanning electron microscopy. (b) Higher magnification view of the area in the rectangle in (a). (c-d) Merged SEM and immunofluorescence images shows paxillin association with podosome radial actin fibers, reaching up to but not co-localizing with the central bundle.
During osteoclast maturation, podosomes cluster together, ultimately forming a wide belt (“sealing zone”) that seals the gap between the cell and the matrix, thus defining the region of bone that is that is earmarked for degradation (Figure 2).

Figure 2
Spatial distribution of sealing zone molecular components. (a) The sealing zone is composed of an actin belt surrounded by inner and outer plaque proteins (e.g. paxillin (b) and phospho-paxillin (c)). (d) On a glass substrate, the sealing zone may expand up to the edges of the multinucleated osteoclast. (e-f) Ratio images of actin/paxillin (e) and paxillin/phospho-paxillin (f) demonstrate that tyrosine phosphorylation is predominantly associated with the plaque domain.
Our research addresses the molecular composition of podosome adhesion and their dynamic formation and dissociation; the effects of surface chemistry and microtopography on osteoclast maturation; the interplay between different cytoskeletal components and podosomes; the regulation of sealing zone stability and dynamics.
Further Reading
Anderegg, F; Geblinger, D; Horvath, P; Charnley, M; Textor, M; Addadi, L; Geiger, B (2011).
Substrate adhesion regulates sealing zone architecture and dynamics in cultured osteoclasts.
PLoS ONE.
6
(12):e28583.


Geblinger, D; Addadi, L; Geiger, B (2010).
Nano-topography sensing by osteoclasts.
Journal of Cell Science.
123
(9):1503-1510.


Luxenburg, C; Geblinger, D; Klein, E; Anderson, K; Hanein, D; Geiger, B; Addadi, L (2007).
The architecture of the adhesive apparatus of cultured osteoclasts: From podosome formation to sealing zone assembly.
PLoS ONE.
2
(1):e179.


- Scientific Activities
- Nano-architecture of adhesion complexes
- Cell-adhesion sensing of the extracellular matrix
- Cancer adhesion and invasion
- Mechanosensitivity of integrin adhesions
- Cell biology of osteoclasts
- Adhesion-mediated signaling
- Platelet adhesion
- Quantitative automated microscopy for high-throughput screening
- Adhesion diversity and the integrin adhesome network
- Adhesion of immune cells
- Heart muscle development
- Publications
- Group Members
- Collaborations
- Screening Projects
- Software
- Galleries
- Research
