Cellular destruction processes are essential for the survival and wellbeing of all cells, tissues, and organs in virtually all organisms. The pathogenesis of many diseases, including cancer and neurodegenerative disorders is attributed to the malfunctioning of these processes. Whereas the use of cell biology and biochemistry approaches have shed invaluable light on some of the basic mechanisms involved in the regulation and execution of different cellular destruction processes, relatively much less is known about the roles and functions of these processes in development and homeostasis of the organism. One of the reasons for the slow progress in this area is the lack of appropriate physiological models available to study cellular destruction processes.
In our lab, we established and investigate three distinct, evolutionarily conserved cellular destruction processes, all associated with the sperm life cycle in Drosophila. These cellular destruction processes range from programmed cell death, the ultimate cell destruction process, to partial elimination of cellular structures during cell remodeling, to selective targeting and elimination of unwanted organelles. While all these processes lead to elimination of cellular structures in essentially the very same cells, the emerging underlying mechanisms appear to be remarkably distinct, consistent with the different levels of destruction required for each process.
The first process, termed germ cell death (GCD), eliminates about 25% of the pre-meiotic germ cells through a caspase-independent alternative cell death (ACD) pathway. ACD pathways received much attention recently, albeit they are mainly investigated in cell culture and the physiological relevance is still in question. Therefore, GCD in flies allows for thorough genetic investigations of the mechanisms and components associated with physiological ACD pathways.
In the second process, the bulk cytoplasmic contents of the terminally differentiating spermatids are normally removed in a process which involves active apoptotic caspases. To date, we know about more than 50 caspase-dependent vital cellular processes in diverse organisms. However, how these cells avoid the lethal activity of caspases and what roles caspases play to promote these cellular processes remains poorly understood. The fly sperm system is perhaps one of the most prominent models to study and address these as well as similar unresolved questions.
The third process selectively eliminates the sperm mitochondria after fertilization. Essentially all organisms inherit their mitochondria maternally but the underlying mechanisms used to eliminate the paternal mitochondria remain controversial. Similar to mammals, the sperm cell in Drosophila contains a flagellum, in which the mitochondria are integral components. These anatomical similarities allow the use of Drosophila as an excellent model for discovering new evolutionary conserved mechanisms and components associated with paternal mitochondrial destruction (PMD). In addition, PMD also serves as a robust physiological model for mitochondrial turnover.
We use a variety of approaches to investigate these CDPs, as well as other related processes, including functional genetics, biochemistry, molecular and cell biology and omics methodologies.

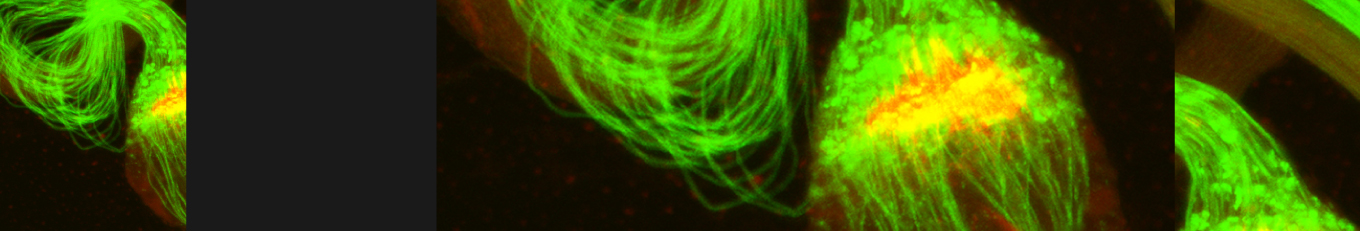
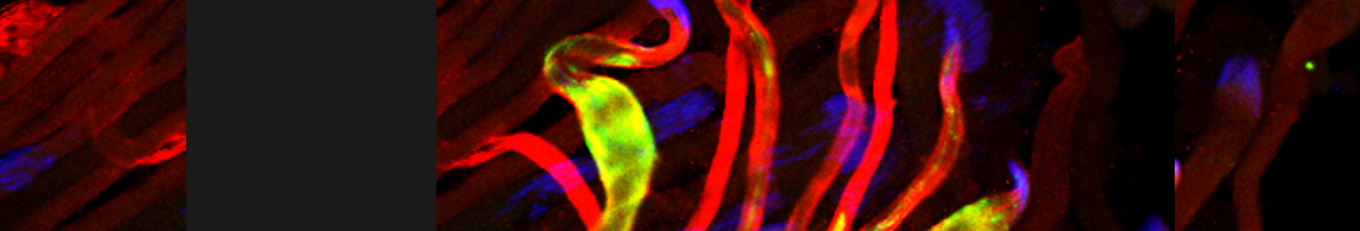
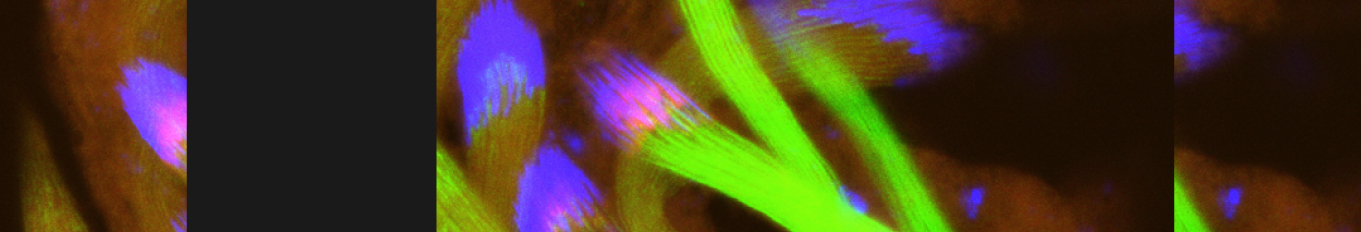
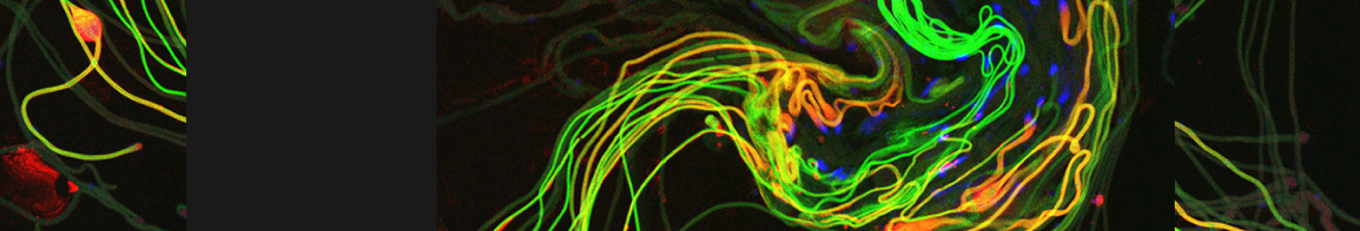
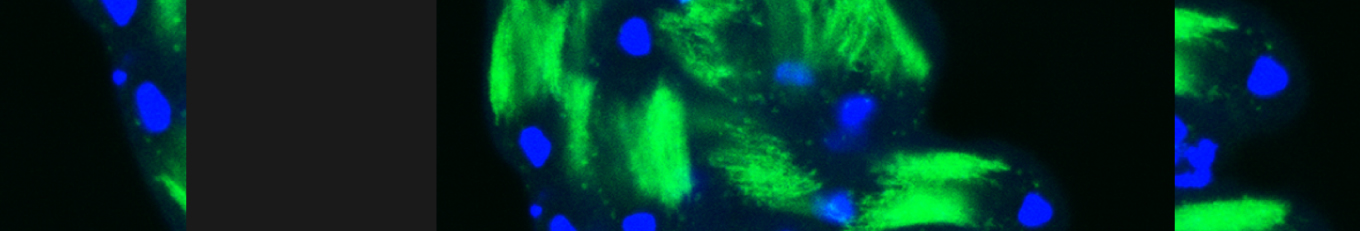
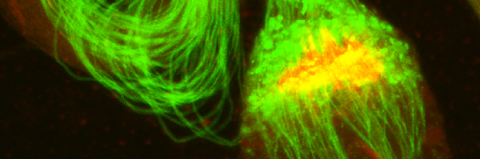
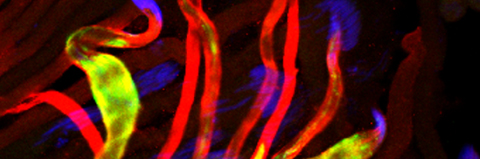
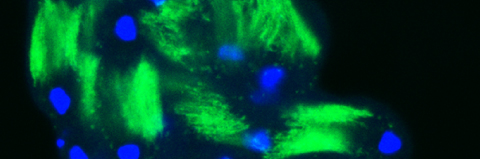
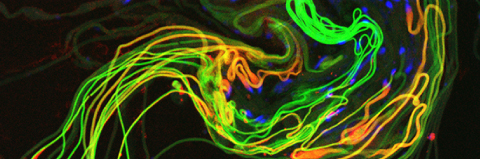
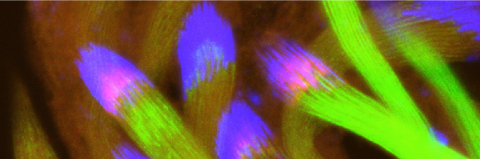
Cellular destruction processes associated with the life cycle of the fly sperm. The different processes are indicated by distinct text colors: A GCD (Germ Cell Death). Shown is the tip region of a testis from wild type strain. Spermatogonial cells are detected by DAPI staining of the nuclei (blue) and expression of the CD8-GFP cell membrane transgene (green). Dying spermatogonia are labeled with TUNEL (red). B The caspase dependent vital cellular process of spermatid individualization. Shown is a ‘cystic bulge’ (CB), which contains the extruded spermatids’ cytoplasmic contents. Spermatids are visualized using an SCS-βA-GFP transgene which marks the mitochondrial derivatives (note that each cyst contains a bundle of 64 spermatids). The individualization complex (IC) which drives the translocation of the CB along the length of the spermatids is stained with Phalloidin (red). C Paternal mitochondrial destruction after fertilization. A sperm mitochondrial derivative is visualized inside an early fertilized egg by crossing wild-type females to male flies expressing the mito-DsRed transgene in spermatids.