Throughout the years, we isolated and characterized six novel soluble receptors (TNF receptors – the TBPI and TBPII, IL-6R, Interferon-γR, LDLR, Interferon α/βR) and three unexpected binding proteins, the IL-18BP, the IL-32BP/PR3 and the heparanase binding protein. Each of those not only contributed to basic science but some also had been translated into clinically useful therapies. Interferon-β was developed as a drug known as Rebif™, which is used for the treatment of multiple sclerosis, while one of the soluble TNF receptors was developed as a drug known as Enbrel™ and is used by rheumatoid arthritis patients. (Novick D. and Rubinstein M. The tale of soluble receptors and binding proteins: from bench to bedside. Cytokine Growth Factor Rev. 18:525, 2007).
I added to this list the well known soluble IL-1 receptor type II (a decoy receptor) using IL-1β sepharose beads. The soluble IL-1 receptor type I and type II were isolated from human urine employing IL-1 receptor antagonist sepharose beads (unpublished data). Elution fractions from these columns were identified by mass spectrometry.
In addition employing ligand affinity chromatography followed by mass spectrometry I also showed that heparanase precursor interacts with resistin, a very surprising interaction. Both are normal body constituent (Plos One, 9 (1):e85944, 2014).
Throughout the years, we raised unique monoclonal antibodies to the various cytokines, their soluble receptors and binding proteins. Our ELISAs enabled monitoring the levels of these biomarkers in health and disease in collaboration with scientists and clinicians from all over the world. Most importantly, based on these ELISAs and the law of mass action, I introduced a calculation of the level of a free cytokine present in body fluids of these patients. Free circulating cytokine is probably what contributes most to the relevant pathology.
Discovery of the soluble TNF Receptors (TBPI and TBPII)-a story of serendipity
(J. Biol. Chem. 265, 1531, 1990)
An attempt to replace the laborious purification of TBPI on several chemical columns with a simple and efficient method, the ligand-affinity column, ended up with a big surprise, the TBPII. The eluate, further resolved by HPLC, yielded two TNF binding proteins, the TBPI and the TBPII (Fig. 1A and 1B). The TBPII (now the drug Enbrel™) would have never been discovered by the multistep chromatographic procedure used beforehand to purify the TBPI, since its chemistry is different from that of TBPI and the fraction containing the TBPII was discarded.
This project was performed in collaboration with Prof. D. Wallach and Dr. H. Engelmann.
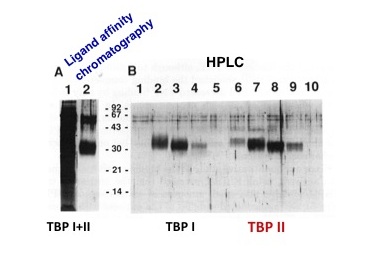
Fig. 1
Discovery of the soluble IL-6 Receptor and soluble Interferon-γ Receptor (Fig. 2A and 2B)- Proof of concept
(J. Exp. Med. 170, 1409, 1989)
Employing ligand affinity chromatography we isolated the soluble interferon-γ receptor, an example of an antagonist, and the soluble IL-6 receptor, an example for an agonist . Thus we showed that soluble cytokine receptors seem to be a general phenomenon reflecting normal constituents of body fluids.
Discovery of the soluble LDL Receptor
(Science, 262, 250, 1993)
(Proc Natl Acad Sci U S A, 110, 7306, 2013)
In 1993 Dr. Dina Fischer in our laboratory identified an antiviral protein in culture supernatants of Interferon-γ induced cells. N-terminal sequencing identified this protein as a soluble fragment of the the extracellular domain of the LDL receptor. I proved that the in-vitro identified soluble LDLR is present also in-vivo as yet another normal body constituent by isolating it from human urine using immunoaffinity chromatography. Recently Dr. Danit Finkelshtein and other members of our group deciphered the mechanism of action of this molecule’s antiviral activity. We found that the cell surface LDL receptor is the entry port of vesicular stomatitis virus (VSV), a virus widely used in gene therapy.
Discovery of the Type I Interferon Receptor
(Cell 77, 391, 1994)
Type I interferon was discovered in 1957 and for several decades, many investigators all over the world searched for its receptor. My discovery of the soluble form of the IFNα/β receptor (Fig. 2C) paved the way to the isolation, characterization and molecular cloning of the ligand-binding chain of its cell surface counterpart (Fig. 2D) which was later on named IFNAR2. This achievement is cited in Immunity 2006 by a world wide known scientist, Prof. Jan Vilcek from NYU, as a milestone of the last 55 years in interferon research.
In addition, we studied the mechanism of action of the Type I interferon and were the first to show that the ligand binding chain of the IFNα/β receptor associates with the cytoplasmic tyrosine kinase JAK1 and thus participates in interferon signal transduction. We also showed a stepwise interaction of interferon with its cell surface receptors. Thus, IFN-α or IFN-β first bind IFNAR2 (the ligand binding chain) to form an intermediate dimer, which then binds and forms a high affinity ternary complex with the accessory chain IFNAR1 (Mol. Cell Biol. 15, 4208, 1995).
Discovery of the Interleukin-18 Binding Protein (IL-18BP), additional example for serendipity
(Immunity 10, 127, 1999)
IL-18 is a pro-inflamatory cytokine first named Interferon gamma Inducing Factor (IGIF). In an attempt to isolate its soluble receptor we discovered the IL-18 Binding Protein (Fig. 2E). IL-18BP is a protein that deviates from the “classical” definition of soluble receptors, as it is not homologous to the extra-cellular portion of the receptor, but is rather coded by a separate gene. We further characterized this protein in vitro and in vivo. This project was done in collaboration with Prof. Dinarello (University of Denver Colorado) and Dr. Soo Hyun Kim. Dr. Vladimir Hurgin in our group characterized the promoter of IL-18BP (PNAS 99, 16957, 2002).
IL-18BP, an extremely potent antagonist of IL-18 and a regulator of mainly Th1 responses, saved recently the life of a baby born with a mutation in the inflammasome which causes overexpression of IL-18. IL-18BP is currently tested in Phase II and phase III clinical studies.
Discovery of the Interleukin-32α Binding Protein (IL-32BP), the enzyme Proteinase 3
(PNAS 103, 3316, 2006)
IL-32 ligand affinity chromatography followed by mass spectrometry yielded IL-32 binding protein (Fig. 2F). This molecule is not a classical soluble receptor but rather a protein with a dual function. On the one hand it is an enzyme, the Proteinase 3 (PR3), and on the other it is an independent cytokine-binding-protein. PR3 is also a known autoantigen in the Wegener’s granulomatosis autoimmune disease and other small vasculitis diseases. (This project was done in collaboration with Prof. Dinarello and Dr. Soo Hyun Kim, Denver University).
Discovery of Heparanase Binding Protein, the Resistin
In an attempt to isolate a heparanase receptor, postulated to mediate non-enzymatic functions of the heparanase protein, we used once again concentrated human urine as a rich source of proteins and affinity chromatography as a specific isolation method. This approach yielded resistin, a very surprising heparanase binding protein. Co-immunoprecipitation, ELISA and a bioassay further confirmed the interaction between heparanase and resistin. It is thus conceivable that this newly identified complex of heparanase and resistin exerts a stimulatory effect also in various inflammatory or other conditions known to be affected by the two proteins. (This work was done in collaboration with Prof. Israel Vlodavsky and Dr. Neta Ilan from the Technion, Haifa).
(Plos One, 9 (1):e85944, 2014)
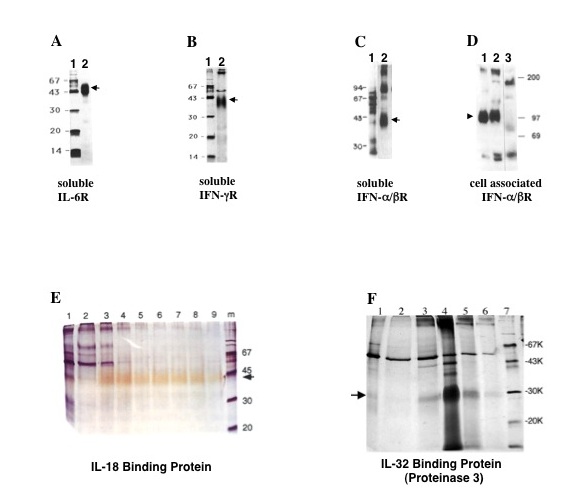
Fig. 2
Summary
The concept of “proteome” was introduced more than 10 years ago, followed by large-scale studies of protein expression, localization, activities and interactions, leading to extensive research and technology development. Proteomics is expansively applied in many areas, ranging from basic research, various diseases and tumors diagnostic and biomarker discovery, to therapeutic applications. Several proteomics approaches have been developed for protein separation and identification, and for the characterization of protein function and structure. Affinity chromatography and mass spectrometry are among the indispensable methods that enabled this meteoric progress. Employing these methods we purified 8 soluble receptors and 3 unexpected binding protein.
Note: The research group was headed by Prof. Menachem Rubinstein