My approach of combining an enriched source of proteins (500-1000 fold concentrated normal human urine) together with highly specific isolation method, the ligand affinity chromatography (Novick, D; Rubinstein, M., Ligand Affinity Chromatography, an Indispensable Method for the Purification of Soluble Cytokine Receptors and Binding Proteins. Cytokine Protocols 2012, 820:195-214), enabled a rapid and efficient isolation of not only soluble receptors corresponding to cell-associated receptors, but also independent binding proteins and associated enzymes (Novick, D; Rubinstein, M., The tale of soluble receptors and binding proteins: From bench to bedside. Cytokine & Growth Factor Reviews. 2007, 18:525-533).
Each round of affinity purification consisted of an equivalent of 250-500 liters of normal human urine concentrated up to 1000 fold, that was passed through a corresponding ligand-affinity column and yielded a specific protein in microgram amounts. The peak elution fractions from these affinity columns were subjected to Edman degredation and in the last 15 years to Mass Spectrometry for the identification of the binding protein.
Ligand affinity chromatography separation is based on unique interaction between the target analyte and a ligand, which is coupled covalently to a resin. It is simple, rapid, selective and efficient purification procedure of proteins providing tens of thousands fold purification in one step. The biological activity of the isolated proteins is retained in most cases thus function is revealed concomitantly with the isolation. Prior to the completion of the genome project this method facilitated rapid and reliable cloning of the corresponding gene. Upon completion of the genome project, a partial protein sequence is enough for retrieving its complete mRNA and hence its complete protein sequence. The ligand affinity chromatography method is indispensable for the isolation of both expected (e.g. receptors) but mainly unexpected, unpredicted and very much surprising binding proteins. No other approach would yield the latter.
The concept of “proteome” was introduced more than 10 years ago, followed by large-scale studies of protein expression, localization, activities and interactions, leading to extensive research and technology development. Proteomics is expansively applied in many areas, ranging from basic research, various disease and tumors diagnostic and biomarker discovery, to therapeutic applications. Several proteomics approaches have been developed for protein separation and identification, and for the characterization of protein function and structure. Affinity chromatography and mass spectrometry are among the indispensable methods that enabled this meteoric progress. Employing these methods we purified 8 novel soluble receptors and 3 unexpected binding protein.
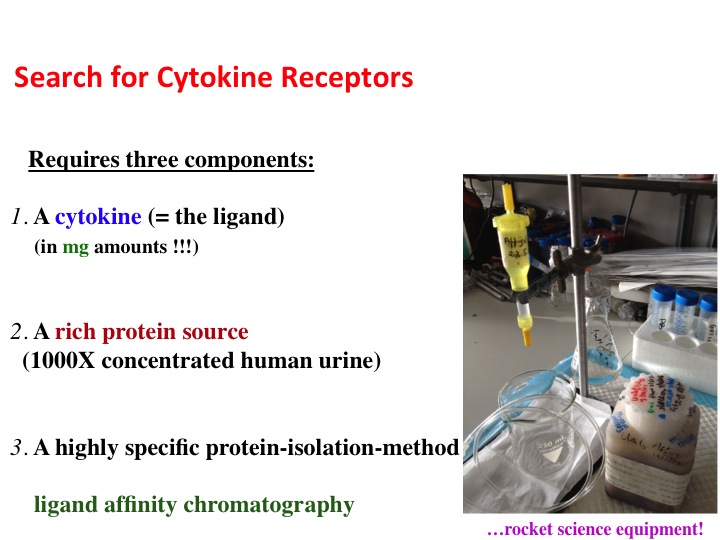
Search for cytokine receptors

Concentrated human urine collection