The 'tailed' heteromeric molecules are the most physiologically important forms of AChE, and the predominant forms in brain and at neuromuscular junctions. Massoulie and colleagues pinpointed a small proline-rich attachment domain (PRAD), around which the globular subunits assemble to form tetramers. The critical feature of this 17-residue peptide is the presence of three and five consecutive prolines; thus even synthetic polyproline can replace the natural PRAD.
Similarly, a well-conserved 40-residue peptide at the C-terminus of the AChE subunit sufficies to form the same quarternary organization by interacting with PRAD. This portion of AChE contains a series of seven aromatic residues, including three equally spaced Trp residues. Thus, it was named the 'tryptophan amphiphilic tetratmerization' (WAT) domain. We have crystallized a complex of this WAT/PRAD complex (ratio 4:1), with selenomethionine incorporated, and plan to determine its structure using MAD.
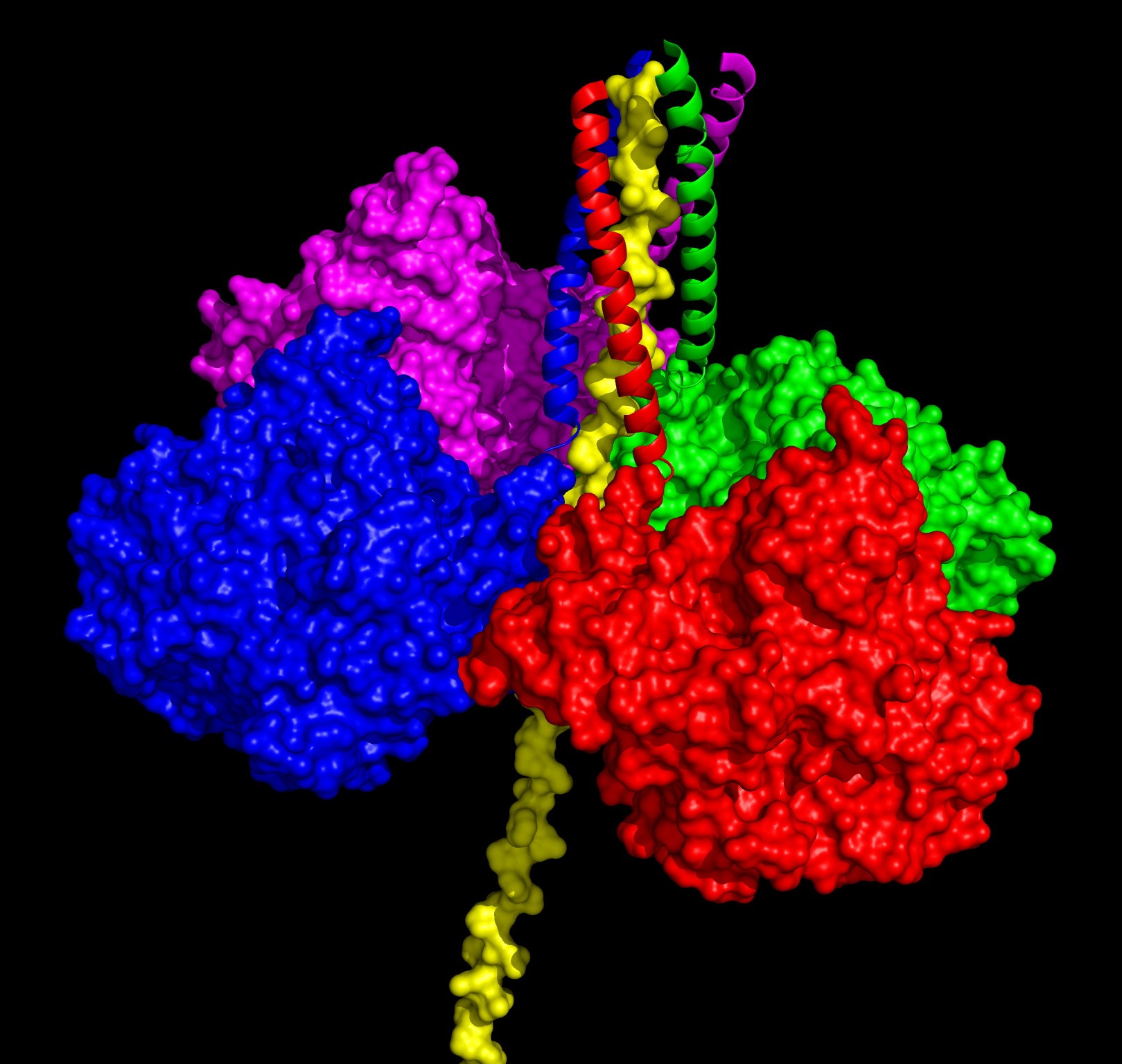
Based on the WAT/PRAD crsystaql structre a full model of the physiological ColQ‐linked AChET tetramer was built. The ColQ polypeptide is vertical. The WAT polypeptides are displayed as ribbons, and the PRAD as a yellow surface model. Subunit color coding is the same as used for WAT chains.


