Dr. Daniel Dar
Food security in a crowded world
New scientists

Fifty years ago, the Earth’s population was three billion people. By 2050, humanity’s ranks will reach nearly 10 billion, increasing global demand for agricultural products at the very time when global warming is threatening farmers’ ability to produce food in sufficient quantities.
Dr. Daniel Dar, a Weizmann-trained scientist who recently joined the Department of Plant and Environmental Sciences, addresses this big challenge with the help of something very small: bacterial colonies that play a surprisingly large role in sustaining modern life.
“To understand bacterial communities, we have to take a deep dive into the complex, microscale world that defines them,” he says, recalling how, in his PhD work under the supervision of Prof. Rotem Sorek of the Department of Molecular Genetics, he examined how human-associated bacteria “sense” antibiotics and mount effective resistance strategies. In his postdoctoral work, however, Dr. Dar focused on bacterial communities that grow on plants—a curiosity-driven line of basic research that could have important implications for agriculture.
“It’s relatively easy to isolate a single bacterium that’s protective against an agricultural pathogen, but until now, we haven’t had the tools to discover what happens when bacteria are put in the ground where plants actually grow a complex environment in which the lab-isolated bacterium comes into contact with a heterogeneous community of microbes,” he says.
Dr. Dar has helped to solve this problem, with an original imaging approach that measures bacterial activities using gene expression, and which he developed together with his postdoctoral advisors at Caltech. The new tool, called par-seqFISH (parallel sequential fluorescence in situ hybridization), makes it possible to track what is happening within bacterial communities in space and time, directly, in their native contexts and at a micrometer resolution. This approach revealed something that could not have been measured until now: that multicellular biofilms are composed of many different and potentially interacting subpopulations, all associated with distinct physiological activities.
Dr. Dar’s experiments were performed on P. aeruginosa, a biofilm-forming bacterium that can cause disease in both plants and animals. His new imaging technique—reported recently in the prestigious journal Science—may be applicable to the study of complex microbial communities in the environment and the human body.
Biosketch
Born in Haifa, Dr. Daniel Dar completed a BSc in biology at Tel Aviv University (2011), then went on to earn his MSc (2018) and PhD (2018) from the Weizmann Institute’s Department of Molecular Genetics. Between 2018 and 2021 he worked as a postdoctoral fellow at the California Institute of Technology.
Dr. Dar is the recipient of numerous awards, including the EMBO Long-Term Postdoctoral Fellowship, the Helen Hay Whitney Foundation Research Fellowship, and the Rothschild Foundation Award (all in 2018), the Giora Yoel Yashinski Prize for Outstanding Doctoral Research (2016), the EMBL Advanced Training Centre Corporate Partnership Programme Fellowship (2015) and the International Union of Microbiological Societies Fellowship (2014).
Married and the father of two, Dr. Dar enjoys playing electric guitar and hiking with his family.
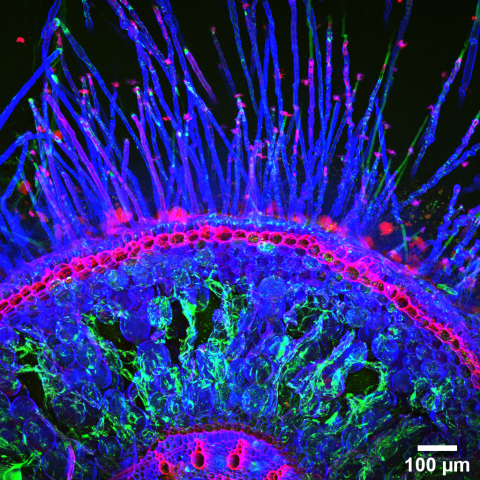
mRNA molecules representing different genes, reflecting different metabolic activities