Turning back the hands of time
The body’s earliest cells are the key to tomorrow’s medicine
Features

When Dr. Jacob Hanna talks about turning back the clock, he is not referring to the latest anti-aging supplement.
He is developing the means to undo time’s arrow in human cells so that they can revert all the way back to their earliest form—the embryonic stem cell state. And then he is moving the hands of the clock forward by just a second or two - to create the cells that naturally appear in the very early embryo and eventually give rise to the sperm and ova. This is what his latest study, published in Cell in December, did - representing the first time that human cells have been programmed into this early developmental stage.
The results of the study will likely help provide answers to fertility problems, yield insights into the earliest stages of embryonic development, and potentially enable the development of new kinds of reproductive technology. This is an exciting horizon. How have we come this far?
Scientists have long understood that mouse embryonic stem cells have the ability to differentiate into any cell type in the body, so their potential for curing disease would appear to be nearly unlimited—if only we could figure out how to get enough of them, and how to ensure they turn into the kind of healthy cells that we want in humans. This is precisely the focus of Dr. Hanna in the Department of Molecular Genetics.
A major boost in the field came in 2006, when Japanese researchers showed that regular adult cells—for example, skin cells—could be “reprogrammed” to an embryonic-like state. The method for creating these socalled “induced pluripotent stem (iPS) cells” involved inserting four genes into the cells. Stem cell researchers worldwide were excited by the findings, but many challenges remained.
For one, the original iPS cells were very similar to embryonic stem cells. However, unlike with mouse iPS cells, human-derived ones retain some traces of “priming” that would direct their differentiation along particular pathways—but just traces. In addition, only around one percent of the treated cells in culture actually tended to revert to a stem cell state and these cells were hard to maintain in the lab.
Thus, the research in Dr. Hanna’s lab has been focused on elucidating how to remove some of the obstacles to creating iPS cells for use. To understand how to create better iPS cells, Dr. Hanna first asked some basic questions: What is preventing the other 99 percent of the cells from reverting to the stem cell state? Why is it that whereas mouse embryonic stem cells are relatively stable in the lab, their human counterparts - induced or natural - are notoriously hard to maintain and become primed and restricted in their potential?
Comparing mouse embryonic stem cells with human iPS cells gave his research group some insights into the genetic pathways in the iPS cells that induce differentiation. “The four genes turn many processes ‘on,’” says Dr. Hanna. “But to get to the true, embryonic stem cell state, other things needed to be turned ‘off’.” They managed to turn those switches ‘off’, and went ahead and successfully created cells that they termed “naïve” iPS cells, which they showed were viable. They also devised a method that could revert many more of the adult cells—up to 100 percent - to an iPS state.
Recreating the feat in human cells
Directing the differentiation and development of iPS cells is, of course, the ultimate goal of stem cell research—and could lead to major advancements in tissue repair and replacement, fertility, and possibly, even in cancer treatment. Dr. Hanna came to this avenue of research after attaining a medical degree and thereby grasping the real needs in medicine.
Dr. Hanna’s postdoc fellowship was done at one of the world’s leading stem cell labs, at MIT; since his return to Israel and joining the Weizmann Institute in 2011, he has established a robust lab that has generated a string of key findings. Such advancements, he says, could not be possible without donor support, for instance that of Ilana and Pascal Mantoux who have sponsored his lab.
Another key breakthrough in the field came in 2011, when a Japanese research team had succeeded in creating primordial germ cells in mice. Once the news of their methods got out, says Dr. Hanna, “the race was on to create them from human cells.” But once again, what worked in the mouse cells did not quite work in the human ones.
Three years later came Dr. Hanna’s latest discovery, the generation of human primordial cells. These cells, which eventually can give rise to sperm and ova, appear within the first week or so of gestation. He created naïve mouse-like iPS cells according to their method, and applied the protocols the Japanese team had used on the mouse cells. It worked: The fluorescent marker his team had added to the genomes of the cells showed that some 40 percent of the cells in the lab cultures had become primordial germ cells.
Investigating further, Dr. Hanna’s team discovered a new genetic pathway controlling the process - different from the one that had been identified in mice. This significant finding raises a number of fresh questions for stem cell researchers who aim to direct the differentiation of human cells.
“For now, the quantity of primordial germ cells we obtained gives us a good number to study, so we can continue our research,” says Dr. Hanna. “There is still a lot to do before we can create sperm or ova in the lab. But one day, we will be able to use this technology to help women who want to conceive after chemotherapy or help men with fertility problems.
He continues, “It is by asking the most basic questions - why human stem cells behave as they do, why they quickly lose their capabilities in conventional growth settings, or what drives them to differentiate - that we will ultimately be able to employ them for our own use.”
Dr. Jacob Hanna is funded by Abisch Frenkel Foundation for the Promotion of Life Sciences, The Benoziyo Endowment Fund for the Advancement of Science, The Sir Charles Clore Research Prize, Erica A. Drake and Robert Drake, European Research Council, Flight Attendant Medical Reseach Institute (FAMRI), Leona M. and Harry B. Helmsley Charitable Trust, Pascal and Ilana Mantoux, France/Israel, Alice & Jacob K. Rubin Charitable Remainder Unitrust, Fritz Thyssen Stiftung. Dr. Hanna is the recipient of the Helen and Martin Kimmel Award for Innovative Investigation.
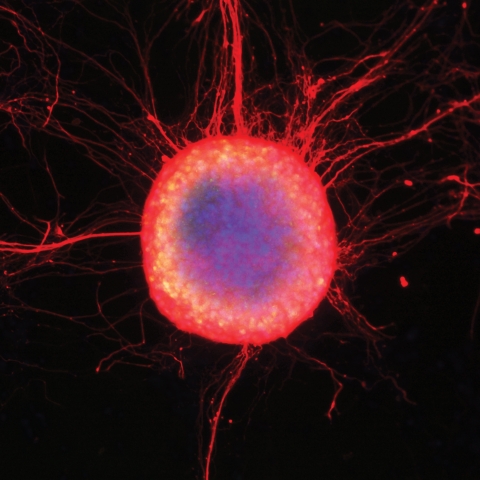
A colony of human embryonic stem cells differentiating into neuronal cells with long extending axons

Dr. Jacob Hanna