The ubiquitin-proteasome pathway in oocytes resuming meiosis
Inbal (Kirenberg) Ben-Eliezer
Oocyte meiosis is a complex process, which consists of two consecutive divisions and involves a large variety of control elements. Chromosome condensation and their segregation are regulated by CDK1, the activity of which is determined by the availability of cyclin B1, which is modified manner throughout the cell cycle in a cycling. Lowering cyclin B1 levels is executed by the ubiquitin-dependent proteolysis machinery that has an essential role in meiosis.
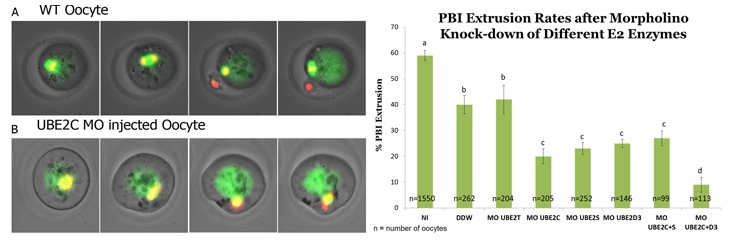
This ubiquitination process consists of three sequential steps facilitated by multiple enzymes- E1, E2 and E3. We specifically aim at unveiling the ubiquitin-conjugating enzymes (E2s) that play a role in this system. For this purpose, GV oocytes were microinjected with morpholino antisense for interference with the translation of specific E2 mRNAs. The microinjected oocytes were allowed to resume meiosis in vitro and microscopically monitored for the extrusion of the first polar body (PBI).
We revealed that by knocking-down any of the three E2 proteins, UBE2C, UBE2S and UBE2D3 the PBI extrusion was inhibited and that spindle formation and chromosome segregation were disturbed. We also found that UBE2C and UBE2S work on the same pathway, while UBE2D3 works on a different pathway. The results of this project shed light on our knowledge of the complexity of meiosis and may potentially contribute to our understanding of unfaithful chromosome segregation.