 |
Yitzhak Koch
Gonadotropin-releasing hormone (GnRH): a brain hormone and a breast hormone |
|---|
 |
Yitzhak Koch
Gonadotropin-releasing hormone (GnRH): a brain hormone and a breast hormone |
|---|
The Adlai E. Stevenson III Professorial Chair of Endocrinology & Reproductive Biology
Tel: (972-8) 934-2790 / 3658
Fax: (972-8) 934-4131
e-mail: y.koch@weizmann.ac.il
After completing my M.Sc. (1963) and my Ph.D. (1969) studies at the Hebrew University of Jerusalem, I moved to Michigan State University (East Lansing) to gain training and experience in neuroendocrinology. I joined the Weizmann Institute in 1971. Since then I have spent two terms of one year as a visiting scientist, first at the National Institutes of Health (Bethesda, MD) and later at the National Institute of Environmental Health Sciences (Research Triangle Park, NC).
The objectives of our laboratory are: (i) to elucidate the biological activities of GnRH, transmitted by milk, on the suckling newborn; (ii) to study the regulation of the expression of the GnRH gene in the mammary gland, as compared to that in the hypothalamus; (iii) to examine our hypothesis that GnRH may exert a direct effect on breast cancer; (iv) to further explore the possibility that the activity of some GnRH degrading enzymes in the pituitary is hormonally regulated, and that the enzymatic machinery in the mammary gland is different; (v) to design and develop novel GnRH analogs that will be useful as contraceptives and as anti-tumor agents.
Analogs of GnRH are currently being tested as a means for the treatment of breast cancer. It is assumed that the anti-carcinogenous effect of these analogs is indirect, and is due to reduced blood levels of estrogen which are caused by desensitization of the pituitary gland to the prolonged stimulation with the GnRH analogs. Our hypothesis assumes, however, that GnRH may have a direct effect on breast cancer. Thus, GnRH that is produced in the breast may also affect the breast in a paracrine and/or autocrine pattern. Recent experiments in our laboratory(6) have demonstrated that the GnRH-receptor gene is transcribed in all mammary gland preparations that we have used. Amplification of the entire coding region of the rat mammary gland GnRH receptor followed by its sequencing have demonstrated that the sequence of the GnRH-receptor cloned from the mammary gland is identical to that of the pituitary gland. Nevertheless, we could not demonstrate high affinity binding sites in the intact mammary gland. It is possible that the different pharmacological properties of the GnRH-receptor in the breast tissue versus the pituitary gland are due to different post-transcriptional modifications such as glycosylation. Another possibility is that the degradation-resistant GnRH analogs that are useful to probe the pituitary receptor are not suitable for the mammary receptor. Indeed, we have recently demonstrated (Leibovitz et al., in preparation) that the specific activity of the mammary gland GnRH-degrading enzymes is far higher than that of the pituitary and, in addition, the pattern of GnRH degradation by the two tissues is different.
Since GnRH acts as a neurotransmitter and because the pulsatile secretion of GnRH is crucial for the proper responsiveness of the pituitary gland, an efficient mechanism for rapid clearance of GnRH is necessary. Peptidases that degrade GnRH have been shown by our group to be present in the cytosolic and membranal fractions of the pituitary gland. We have demonstrated earlier that analogs of GnRH that are modified at the 6th position of the neurohormone are less susceptible to degradation by pituitary and hypothalamic enzymes. Since such GnRH agonists also exhibit higher affinity to the neurohormone receptor, it may be assumed that resistance to degradation, as well as higher receptor binding affinity, contribute to the increased biological activity of these analogs. In recent studies, we have found that long-term desensitization of female rats, induced by subcutaneous implantation of a slow-release preparation of buserelin, a GnRH analog, for 21 days, resulted in a dramatic increase in the activity of the cytosolic enzymes that degrade GnRH, and some of the primary degradation products underwent secondary degradation(7). On the other hand, a significant decrease in the activity of the cell surface enzymes that degrade GnRH was found. Thus, desensitization of the pituitary toward GnRH is accompanied by substantial changes in the activity of enzymes that are responsible for the neurohormone's clearance.
Gilad, I., Weizman, A., Silbergeld, A., Dickerman, Z., Kaplan, B., Laron, Z., and Koch, Y. Different effect of insulin like growth factor I and growth hormone on hypothalamic regulation of growth hormone sectretions in the rat. J. Endocrinol. Invest. 19: 542-547 (1996).
Levi, L.N., Ben-Aroya, N., Tel-Or, S., Palmon, A., Burstein, Y. and Koch, Y. Expression of the gene for the receptor of gonadotropin-releasing hormone in the rat mammary gland. FEBS Lett. 379: 186-190 (1996).
Leibovitz, D., Fridkin, M., Ben-Aroya, N., and Koch, Y. Degradation of gonadotropin-releasing hormone (GnRH) by pituitary enzymes is modulated by densensitization. Isr. J. Obstet. & Gynecol. 8:151-157 (1997).
Rahimipour, S., Weiner, L., Shrestha-Dawadi, P.B., Bittner, S., Koch, Y. and Fridkin, M. Cytotoxic peptides: Naphtoquinonyl derivatives of luteinizing hormone-releasing hormone (LH-RH). Lett. Peptide Sci. 5:421 (1998).
Chen, A., Yahalom, D., Ben-Aroya, N., Kaganovsky, E., Okon, E. and Koch, Y. A second isoform of gonadotropin-releasing hormone is present in the brain of human and rodents. FEBS Lett. 435:199 (1998).
Yahalom, D., Koch, Y., Ben-Aroya, N. and Fridkin, M. Synthesis and bioactivity of fatty acid-conjugated GnRH derivatives. Life Sci. 64:1543 (1999).
Chen, A., Levy-Laskar, O. and Koch, Y. selective expression of neuropeptides in the rat mammary gland: the somatostatin gene is expressed during lactation. Endocrinology 1999 (in press).
Yahalom, D., Koch, Y., Ben-Aroya, N. and Fridkin, M. Hexapeptide and cyclic pentapeptide endothelin antagonists directly activate pituitary gonadotropin-releasing hormone (GnRH) receptors. (Submitted).
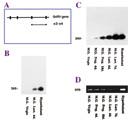 |
(A) A schematic diagram of the GnRH gene. (B, C) This gene is expressed in the hypothalamus and in the mammary glands (M.G.) of lactating (Lact.) rats on days 6 and 7 of lactation, as well as during late pregnancy (18 days), but not in the mammary glands of virgin rats. Another gene (actin) serves as a control (D) to demonstrate that, unlike the GnRH gene, it is expressed in all the samples.
|
|---|