A functional immune system depends on targeted cell migration, which is regulated by the association of chemokines to both chemokine receptors and glycosaminoglycans (GAGs). Chemokines constitute a family of small secreted proteins (8-10 kDa) that regulate leukocyte trafficking to sites of inflammation and cell repair, playing important roles in angiogenesis and metastasis, hallmarks of cancer, and leukocyte development. CCR5 is a chemokine receptor that is the major co-receptor of HIV. CCL5, also known as RANTES is a chemokine ligand of CCR5 involved in leukocyte trafficking and is a natural HIV suppressive factor. The CCR5:CCL5 axis constitutes, therefore, a promising therapeutic avenue for drug development and an active area of research for both experimental and computational structural biology. Using transferred-NOE and isotope-edited/isotope-filtered experiments on complexes in which the chemokine is uniformly labeled with 13C and the CCR5 peptide is unlabeled, we are studying the interactions CCL5 with the N-terminal segment of CCR5 that contains two sulfated tyrosine residues.
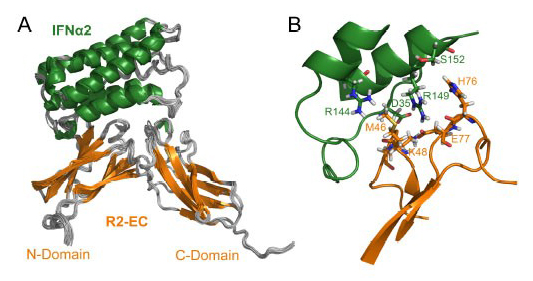
Model of the IFN/R2-EC Complex. A) Ensemble of the 20 lowest energy structures of cluster I of the docking procedure. B) Close-up view of the interface of the complex. IFN is shown in green and R2-EC in orange. Residues involved in double mutant cycle constraints are shown in stick representation and labeled in the same colors as the protein.