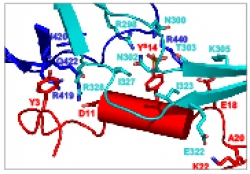
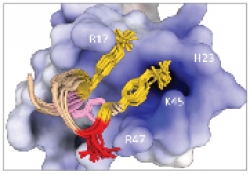
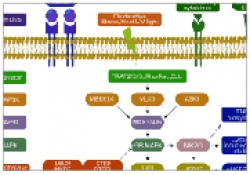
Our Laboratory is studying biological recognition at atomic resolution using solution-state Nuclear Magnetic Resonance (NMR) spectroscopy. The main research topics in the lab are: HIV-1 binding to target cells, chemokines interactions with their receptors and the interactions between key protein molecules involved in signal transduction cascades. In addition to applications of state of the art NMR experiments to structure determination, our group is pioneering in developing methods and approaches to use transferred-NOE to study intermolecular interactions in protein complexes over a wide range of molecular weight from 10 to more than 100 kDa. Research in the lab involves using state of the art multi-dimensional NMR experiments, analysis of NMR data and structure determination, protein expression and labeling in E. coli and mammalian cells and protein purification using liquid chromatography techniques.
Protein-Ligand Interactions by NMR