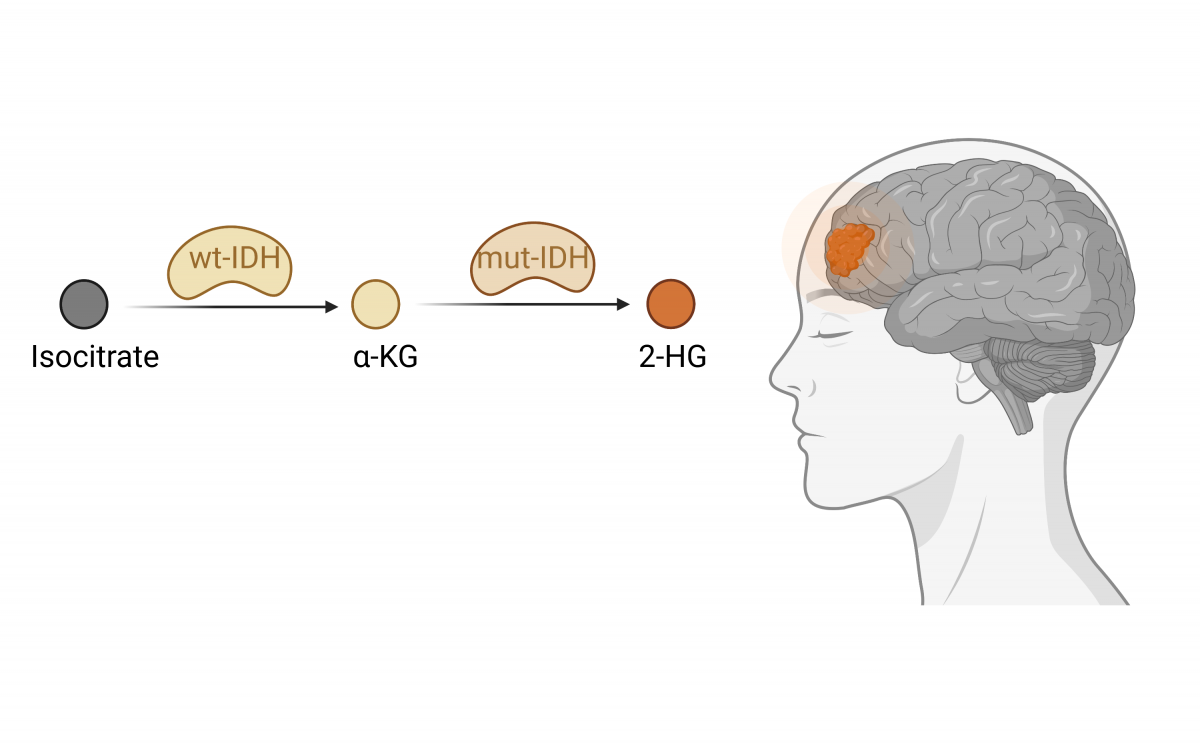
Malignant gliomas are heterogeneous tumors arising in the central nervous system (CNS), highly resistant to anti-cancer treatments. Epigenetic and metabolic aberrations are considered early events during gliomagenesis and often define specific glioma subtypes. Isocitrate dehydrogenase (IDH1/2) enzymes are frequently mutated in adult gliomas, and their mutation status is currently used for CNS tumor classification. These gain-of-function mutations alter IDH activity, favoring the conversion of a-ketoglutarate (α-KG) to the oncometabolite 2-hydroxyglutarate (2-HG), a competitive inhibitor of α-KG-dependent enzymes. Among the inhibited proteins are various epigenetic regulators disrupting the chromatin landscape. Leveraging epigenetic-focused cytometry by time-of-flight (CyTOF) analysis, we profiled the effect of mutant-IDH expression on a broad panel of histone modifications. This high-dimensional analysis reveals the extensive remodeling of chromatin patterns by mutant-IDH. In addition, it highlights novel epigenetic pathways affected by the mutant enzyme and may contribute to its pro-tumorigenic features. We complement this analysis with different methodologies underscoring the tight interaction between chromatin and metabolism dysregulation in glioma.