Home
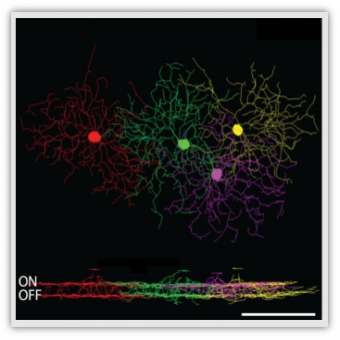
The retina – the neuronal tissue located at the back of our eyes – is where all visual processing begins. The retina splits the visual information into parallel channels, each encodes a specific modality of the visual scene, such as edges or motion. These computations are carried to secondary visual structures by different types of retinal ganglion cells (RGCs) – the output neurons of the retina.
Recently, we and others found that the modality encoded by RGCs—traditionally considered a fixed hardwired property of each RGC type—can be altered under certain conditions. For example, direction selective RGCs reorient their directional tuning following a short repetitive stimulation, and other RGCs change their polarity preference with changes in ambient light levels (On/Off). These newly discovered dramatic changes in the core computations of RGCs depart from the known retinal adaptation that includes gain adjustment but no modality changes. Moreover, they raise a critical question for our understanding of vision: How do neurons downstream to the retina interpret the dynamic retinal code and how, despite such dynamics, a consistent representation of the visual scene emerges?
In the lab, we use state-of-the-art electrophysiology, two-photon imaging, and pharmacogenetic techniques to study the mechanisms involved in retinal dynamics and to resolve how the computations performed by anatomically defined neuronal circuits are altered by external stimulation. In parallel, we investigate the transfer function between the retina to its primary target, the visual thalamus (LGN), to determine how the LGN decodes retinal dynamics.
Using the retina for early diagnostic of neurodegenerative diseases
Parkinson’s disease is caused by death of dopaminergic neurons in the midbrain. The retina contains dopaminergic neurons as well, and there is evidence that retinal dopamine levels are decreased in Parkinsonian patients. We aim to determine how the responses of retinal neurons change as a function of dopamine levels. Our goal is to provide the first step to establishing of a simple visually-based method to diagnose Parkinson’s disease and determine its progression.
The cholinergic system is most severely affected in Alzheimer's disease. The reduction of acetylcholine, found in the cortex and certain subcortical areas, might also occur in the retina. Indeed, visual symptoms are often among the first complaints of patients suffering from Alzheimer's disease, and a substantial loss of retinal neurons is documented in Alzheimer's patients. While the retina is comprised of various types of neurons, it contains only one type of cholinergic neurons, the starburst amacrine cells (SACs). SACs belong to the direction selective circuit, which mediates the optokinetic reflex – a visual reflex involved in tracking motion in the visual field, and that can be easily measured in the lab/clinic. We aim to use direct measurements from retinal neurons, in order to evaluate Retina’s cholinergic levels via the visual reflex. These measurements from retinal neurons may be used for early diagnose of Alzheimer disease based on a simple, non-invasive, visual test.
Funding
Our research is generously supported by grants from the Israely Science Foundation (ISF) and from the European Research Council (ERC) starter grant.



The lab is a member of the Israel Science Foundation's I-CORE Center of Excellence in Cognition.
We are grateful for the generous support of the following foundations:
Charles and David Wolfson Charitable Trust
The Revson Award for Advancing Women in Science
The Peter and Patricia Gruber Awards
Dr. and Mrs. Alan Leshner
The Simon Family Foundation
Ms. Lois Pope
The Lubin-Schupf Fund for Women in Science.
Michal Rivlin is the incumbent of the Sara Lee Schupf family chair.