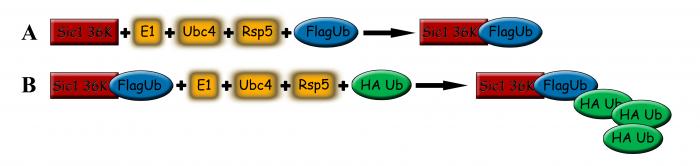
Premature deubiquitination of a proteasomal substrate may interfere with substrate degradation, by dissociating it from the proteasome. Conversely, failure to remove or trim the polyubiquitin chain would prevent further processing and may even clog the proteasome. Thus, as deubiquitination must occur prior to unfolding and translocation into the proteasome catalytic core (20S), a mechanism that keeps the deubiquitinated substrate appended to the proteasome regulatory complex (19S) must exist. We propose that the proteasomal deubiquitinating enzymes (Dubs) spare the substrate proximal ubiquitin molecule, which in turn serves to hold the substrate en route to degradation, and this ubiquitin molecule is degraded along with the substrate.