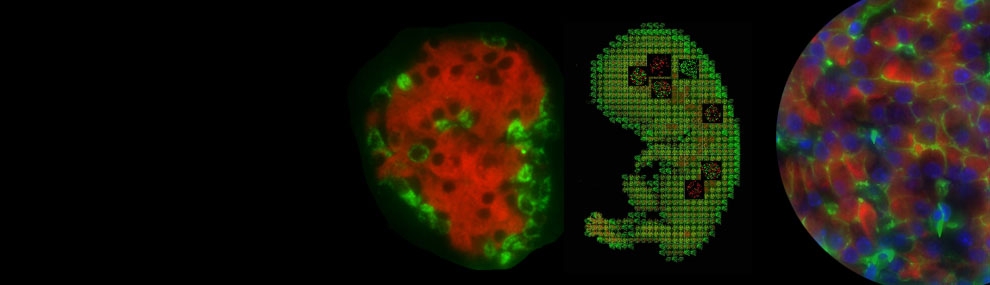
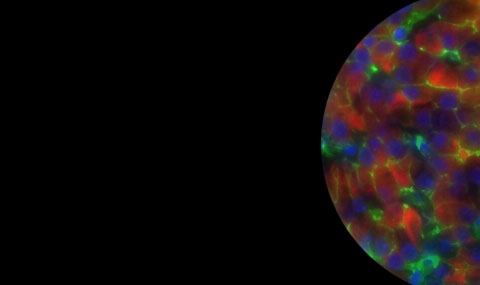
Diabetes mellitus is a life-threatening metabolic disease whose prevalence is rapidly increasing worldwide. It is characterized by elevated blood glucose levels (hyperglycemia) due to insufficient circulating insulin levels. Insulin is secreted from pancreatic β cells that reside in the islets of Langerhans. Islet transplantation has been used to treat Type 1 diabetics. However, this approach is very limited due to the shortage of donor tissue. Alternative approaches for therapy have been suggested, such as cell replacement therapy using insulin-producing cells derived from differentiation of human embryonic stem cells, or the direct reprogramming of somatic non-β cells.
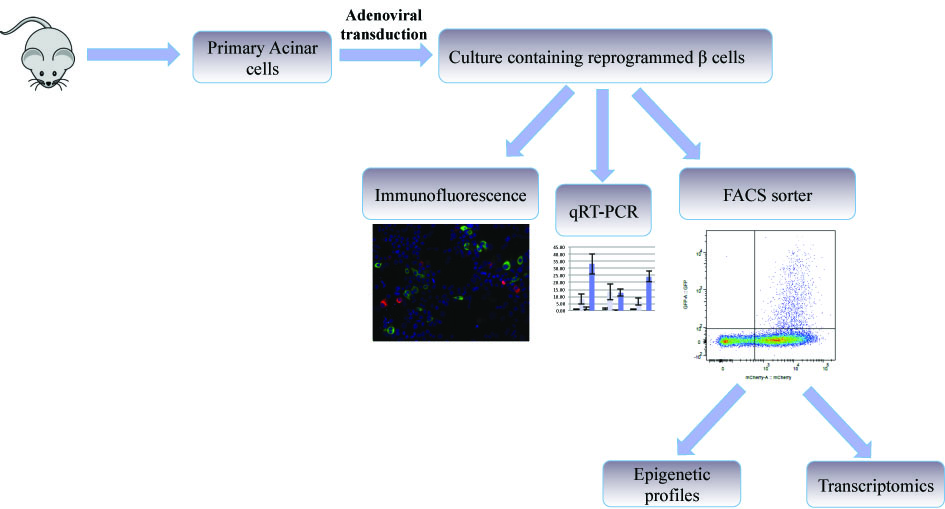
The goal of our research is to clarify the molecular mechanisms involved in the process of reprogramming to beta cells. We have developed an in vitro system that allows the study of reprogrammed β cells originating from primary mouse pancreatic acinar cells.
We have been able to demonstrate reprogramming of pancreatic acinar cells into insulin producing β cells by adenoviral gene delivery of three transcription factors involved in β cell development and function: PDX1, NGN3 and MafA (PNM). Lineage tracing experiments approved the acinar origin of reprogrammed cells. In addition, isolation of primary acinar cells from transgenic mice expressing the GFP reporter under the control of the insulin reporter, enabled FACS purification of reprogrammed cells. We now study extensively the transcriptional and epigenetic profiles of these reprogrammed cells.
Furthermore, by transducing acinar cells with different combinations pancreatic transcription factors, we have been able to direct acinar cell reprogramming to other endocrine lineages additionally to the β cell lineage. We now peruse studies aiming at characterizing the relative roles of different pancreatic transcription factors in directing acinar cell reprogramming to the different endocrine lineages.
This robust in vitro system allows us to study molecular mechanisms underlying reprogramming of the exocrine pancreas into insulin-producing β cells as well as other endocrine cells.
Improved understanding of these processes is essential to permit their application in the future for regenerative and cell therapy-based treatment of diabetes.
Aims of the study
- Characterization of the reprogrammed cell population
- Identification of signaling pathways involved in the reprogramming process toward β cell lineage
- Uncovering how the PNM transcription factors work alone or in concert to generate different pancreatic lineages.