Mending broken hearts
What if heart cells could regenerate?
Briefs
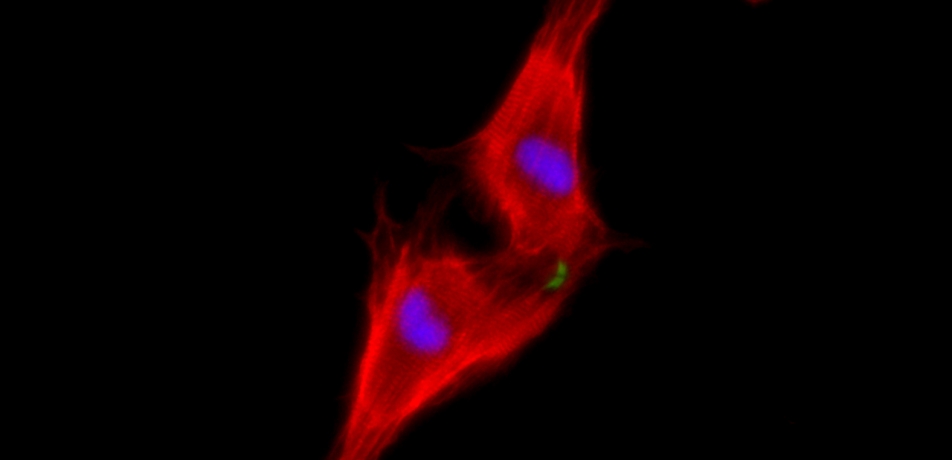
Two neonatal cardiomyocytes (stained red) undergoing cell division after treatment
A bad haircut isn’t doomsday—our hair will always regrow. A child with a fresh gash from a playground incident can be appeased with a mom’s promise that the skin will heal.
And donating blood may be altruistic but, after all, after a day or so our supply has renewed itself and we are back in business. Indeed, blood, hair, and skin cells renew themselves all the time; most of the body’s cells, in fact, contain automatic mechanisms of regeneration.
But heart cells never do, which is why heart disease is so fatal. Prof. Eldad Tzahor wanted to know why not - and see if he could turn around the heart’s fate.
In a study in Nature Cell Biology in April, Prof. Tzahor, an expert in embryonic development with a focus on the heart, found that a protein called ERBB2, in concert with other proteins and protein receptors, controlled - and stymied - the development of heart tissue, so that it was able to grow only up to a week after birth, in mice. He and his colleagues then went on to discover, through various experiments, that ERBB2 - if activated with great precision in a short interval after a heart attack - could nearly completely heal a heart within several weeks.
“The results were amazing,” says Prof. Tzahor. “As opposed to extensive scarring in the control hearts, the ERBB2-expressing hearts had completely returned to their previous state.”
But precision and timing are key. If too much or too little of the protein combination is applied, the results could be devastating on the heart, either by weakening or enlarging it beyond repair.
So how does the regeneration process happen? Through the intervention of the precise quantity and timing of ERBB2, heart muscle cells called cardiomyocytes revert to an earlier form of themselves. In this “undifferentiated” form, the heart muscle cells exist as something between an embryonic and an adult cell, which can then divide and differentiate into new heart cells. And here’s where the precision comes in: a fusion of ERBB2 takes the cells back a step to an earlier, embryonic form; and then stopping its activity promotes the regeneration process.
Prof. Tzahor and his team began to outline the pathway of proteins and messaging systems in the cardiomyocytes, which will elucidate the roles of other proteins in the chain of events. Those proteins may present new drug targets for treating heart disease, says Dr. Gabriele D’Uva, a postdoctoral fellow in the lab, because they affect the activity of ERBB2, primarily a protein receptor called NRG1.
And in an ideal example of how basic research in one field illuminates understanding in another, these insights are also expected to hold meaning for cancer research, because the same pathway is involved in tumor development.
Other scientists involved in this study included Profs. Yosef Yarden and Michal Neeman, also of the Department of Biological Regulation, and Prof. Jonathan Leor of Chaim Sheba Medical Center, and Richard P. Harvey of the University of New South Wales, Australia.
Prof. Eldad Tzahor is supported by the Adelis Foundation, the Wolfson Family Charitable Trust, the European Research Council, the Louis and Fannie Tolz Collaborative Research Project, the estate of Fannie Sherr, and the estate of Jack Gitlitz.
http://www.weizmann.ac.il/mcb/tzahor/#home
Prof. Yosef Yarden is funded by Dr. Miriam and Sheldon G. Adelson Medical Research Foundtion, The Dwek Institute for Cancer Therapy Research, which he heads, Rising Tide Foundation, Marvin Tanner Laboratory for Research on Cancer, Maurice and Vivienne Wohl Biology Endowment. Prof. Yarden is the incumbent of the Harold and Zelda Goldenberg Professorial Chair in Molecular Biology.
http://www.weizmann.ac.il/Biological_Regulation/Yossi_Yarden
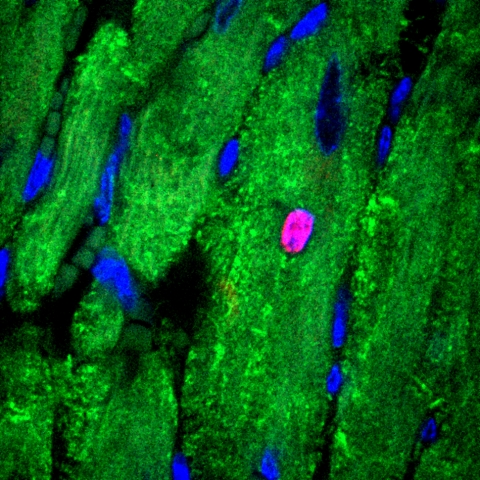
Cell-cycle activity in the cardiac tissue of an adult mouse administered ERBB2
