Bacteria on the run
Prof. Rotem Sorek and the microbial arms race
Features

While he was a postdoctoral researcher, Prof. Rotem Sorek became curious about an odd genetic quirk he noticed when sequencing a large number of genes in bacteria.
He wondered why certain sections of the genes were not sequenced. They seemed to disappear in the sequencing process. He wanted to find out why, and what happened to them.
Prof. Sorek discovered that these gaps are a limitation of the genome sequencing technique itself - not the genome. In the past, sequencing a bacterial (or any other) genome involved cutting up the genome and inserting the pieces into another bacterium - the ubiquitous E. coli, known for causing food poisoning - for duplication and further sequencing. Gaps in the sequence occurred all the time. He discovered that the missing genes encoded toxins that killed the host E. coli cells, and that’s why their DNA was missing. That made him think: Identify the genes that code for toxins that kill E. coli, and you’ve got a potential therapy for bacterial infection. He went on to develop a computerized method that flags these particular bacteria-killing gene sequences.
He joined the Department of Molecular Genetics in 2008, and put his research into full gear. Since then, Prof. Sorek and his lab team have discovered tens of thousands of such bacteria-killing genes from hundreds of bacteria and catalogued them in the PanDaTox database he shares with fellow scientists around the globe. His serendipitous discovery opened wide a new field of microbiology that offers hope for new types of antibiotics and anti-bacterial compounds. In an era in which physicians and scientists alike are wringing their hands about increasing resistance of bacterial infections to antibiotics, this research field is more important than ever. Prof. Sorek has been issued seven patents and has 25 more patent applications pending for his innovations.
Most recently, he has focused on undertanding the interactions between bacteria and the viruses, called phages, that kill them. In a recent study, he detected nearly a thousand gut-associated phage genomes. His discoveries suggest that phages contribute to shaping the composition of bacteria in our gut, and therefore may affect our health. Whereas much research in recent years has focused on bacteria in the gut, this insight indicates that the bacteria are far from alone: they are engaged in an intimate dance with viruses that affect their activity - and the health of the people in whose intestines and stomachs they reside.
A bacterial grudge
Prof. Sorek calls it the “microbial arms race”: how bacteria fight each other as part of their struggle for survival, and how they defend themselves against phages that infect and kill bacteria. Phages are 10-fold more abundant than bacterial cells and are an important part of the ecology of life in the oceans and in the soil, and also in the human gut.
He is one of the leaders in studying a form of adaptive immunity found in bacteria, called CRISPRs. When a virus gets inside a bacterial cell, the host cell steals a small bit of the virus’s DNA and stores it in the form of a genetic piece in its own genome. These tiny bits are known to scientists like Prof. Sorek as CRISPRs. “The process of saving CRISPRs is like holding a grudge against the phage,” he jokes. “The next time the same virus infects the cell, the bacterial genetic immune system will use the stored viral DNA to identify and destroy the invader, much like vaccinations against human diseases in the process known as adaptive immunity.” In addition, scientists have learned how to use the ingenious CRISPR system to “edit” the human genome - making it a handy tool for gene therapy and other applications.
The study of CRISPRs quickly became a hot one in biology, and he went on to make more enlightening discoveries about them. For example, Dr. Sorek and his team recently deciphered the mechanism by which the CRISPR differentiates between the foreign, invading phage and the self-genome. The CRISPR system identifies DNA that replicates rapidly. Thus, the phage’s survival tactic - its programmed drive to replicate at all costs - proves to be its downfall. This has been a major puzzle in the newly developing field.
His further research has revealed a completely new anti-phage defense system, called BREX, which is widespread in bacteria. This system acts like a shotgun, conferring resistance on a broad range of phages. His studies of the bacterial immune system are aimed to uncover other new “weapons” and “shields” that bacteria and viruses use as part of their ongoing warfare, and harness them for the benefit of mankind.
Prof. Rotem Sorek is supported by the Abisch Frenkel Foundation for the Promotion of Life Sciences, the European Research Council, the Leona M. and Harry B. Helmsley Charitable Trust, the Dana and Yossie Hollander Center for Structural Proteomics, the Hugo and Valerie Ramniceanu Foundation, the Dr. Dvora and Haim Teitelbaum Endowment Fund, Mr. Martin Kushner Schnur in honor of Fanny Kushner, and the estate of Samuel and Alwyn J. Weber
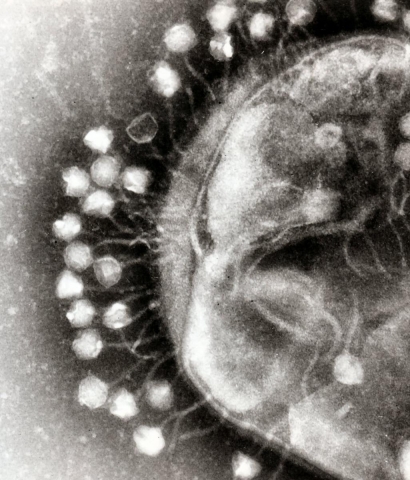
A phage virus

Outside his lab, Prof. Sorek is an avid windsurfer and mountain bike