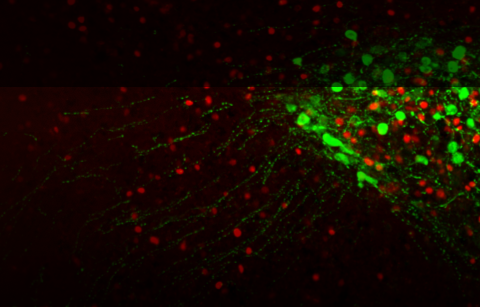
Sexually dimorphic reproductive behaviors such as courtship, mating, nursing, and aggression, are crucial to the reproductive success of the animal and the survival of the species. However, the encoding of these sex-specific behaviors by neural circuits remains a long-standing and fundamental question in neurobiology.
Surprisingly, with a few exceptions, the quest for fundamental differences between the male and female mammalian brains, that would parallel the differences seen in reproductive behaviors, has so far uncovered mostly modest quantitative differences, rather than clear dissociations (Zilkha et al 2016, 2017, 2021).
In this line of research, we have recently targeted a novel region with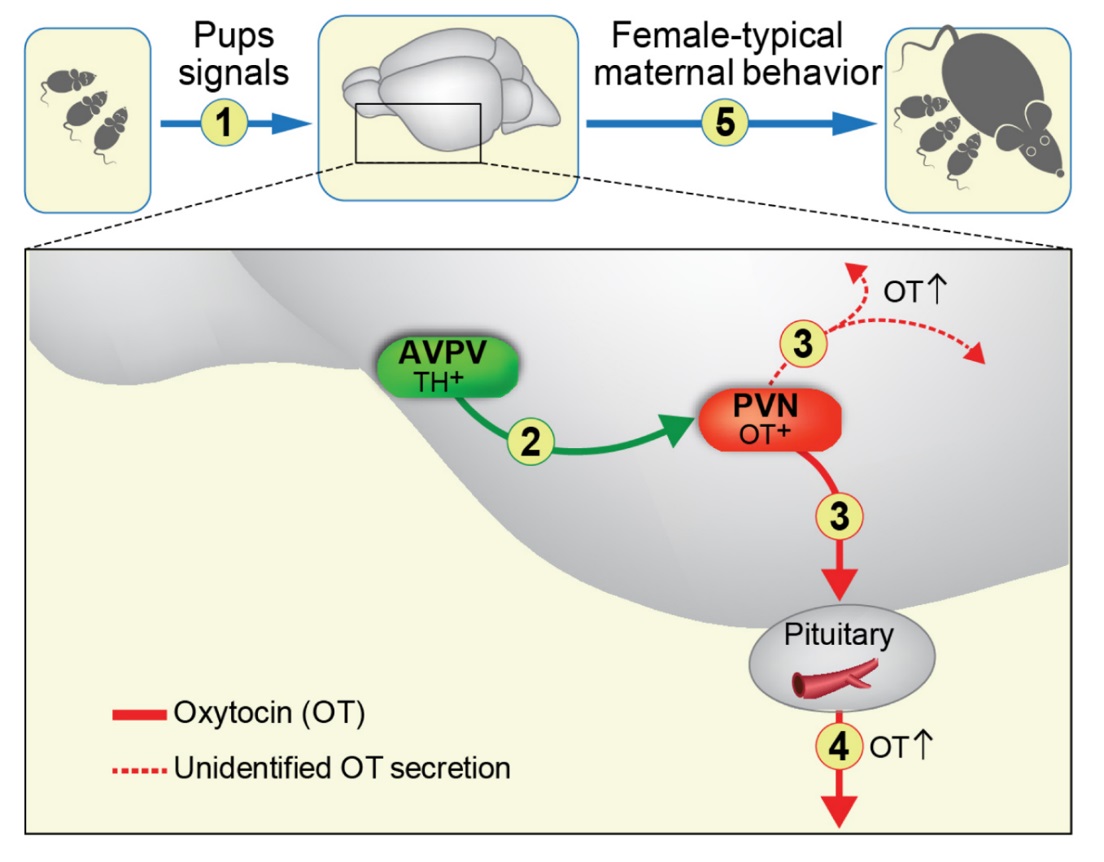 in the hypothalamus, which is both sexually dimorphic and carries a crucial role in maternal care: the anteroventral periventricular nucleus (AVPV) (Scott et al 2015). Specific ablation of Tyrosine-hydroxylase (TH) cells in the AVPV severely impaired maternal care and reduced plasma oxytocin levels, in both virgin and post-partum female mice, while optogenetic activation enhanced maternal behavior and OT secretion. Notably, manipulation of AVPV TH neurons did not affect paternal behavior of males, but rather regulated inter-male aggression (Scott et al 2015).
in the hypothalamus, which is both sexually dimorphic and carries a crucial role in maternal care: the anteroventral periventricular nucleus (AVPV) (Scott et al 2015). Specific ablation of Tyrosine-hydroxylase (TH) cells in the AVPV severely impaired maternal care and reduced plasma oxytocin levels, in both virgin and post-partum female mice, while optogenetic activation enhanced maternal behavior and OT secretion. Notably, manipulation of AVPV TH neurons did not affect paternal behavior of males, but rather regulated inter-male aggression (Scott et al 2015).
In our current research, we explore the role of oxytocin (OT) neurons in the paraventricular nucleus (PVN) of the hypothalamus, in controlling aggression and group organization in male and female wild-derived mice. Our results show that OT+ PVN neurons control aggression and dominance hierarchy in females, but play a minor role in regulating the same phenotypes in males. In agreement with these behavioral findings, we reveal sexual dimorphism in the projection and activation patterns of this neuronal population (Sofer et al., in prep).
- Zilkha N, Sofer Y, Beny Y, Kimchi T. 2016. From classic ethology to modern neuroethology: overcoming the three biases in social behavior research. Current Opinion in Neurobiology 38: 96-108
- Zilkha N, Scott N, Kimchi T. Sexual Dimorphism of Parental Care: From Genes to Behavior. Annu Rev Neurosci. 2017;40:273-305. doi:10.1146/annurev-neuro-072116-031447
- Scott N, Prigge M, Yizhar O, Kimchi T. 2015. A sexually dimorphic hypothalamic circuit controls maternal care and oxytocin secretion. Nature 525: 519-22