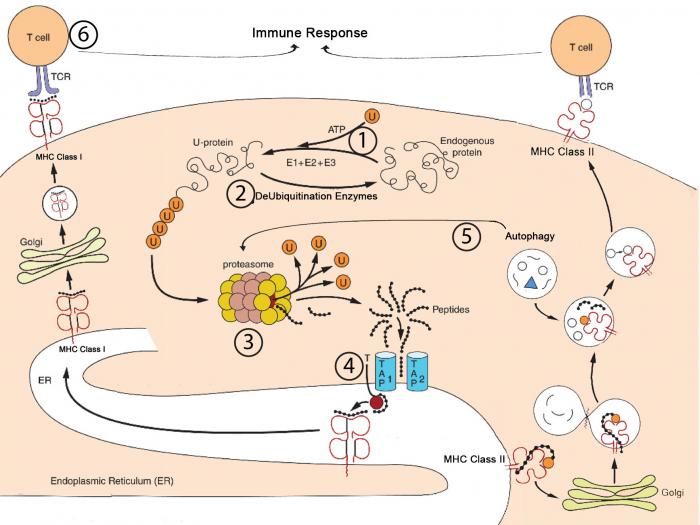
The regulated degradation of proteins within eukaryotic cells is considered to be catalyzed primarily by the ubiquitin – proteasome system. In this system, substrates are targeted for proteasomal degradation by covalent ligation to ubiquitin, in a multi-step process, which requires a sequential action of three enzymes (E1, E2, E3). The 26S proteasome is composed of two major sub-complexes, the 20S catalytic core and the 19S regulatory particle. The 26S proteasome plays an important role in the generation of peptides suitable for binding and presentation by Major Histocompatibility Complex (MHC) class I molecules. The other major cellular recycling pathway, macroautophagy, is responsible for nonspecific bulk degradation of cytoplasmatic components and damaged or excess organelles. A fraction of the lysosomal degradation products are loaded on MHC class II molecules in the endocytic compartment. These two degradation pathways are responsible for the generation of peptides suitable for binding to MHC molecules. On the cell surface the peptide-MHC complex is surveyed by epitope-specific T cell receptors, carried on T lymphocytes; this specific recognition triggers the activation of the immune response.