In vitro model for studying the regulation of oocyte maturation
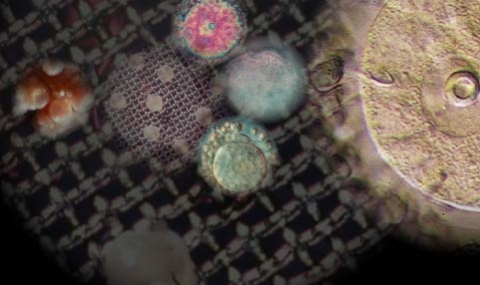
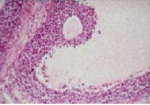
Oocyte meiosis in mammals is a protracted process, subject to several stop-go controls. The oocyte initiates the first meiotic division during embryonic life or shortly after birth. Meiosis is arrested, in most mammals, in neonatal life at diplotene/dictyate stage of the first meiotic prophase. The hallmark of the dictyate oocyte is the large nucleus known as “germinal vesicle” (GV).