Bone & Osteoclasts
Bone is a dynamic tissue constantly built and remodeled through the action of two major cell types: osteoblasts that deposit new bone matrix and osteoclasts that resorb old and damaged bone. The fine balance between bone formation and resorption is essential for physiological bone formation and homeostasis, and disruption of that balance leads to severe pathological states, such as osteoporosis (loss of bone) and osteopetrosis (excessive bone density).
Studies in the Geiger laboratory addressed two main aspects of osteoclast function:
The mechanisms underlying osteoclast adhesion to the underlying surfaces (bone or tissue culture substrate), focus on two major adhesion structures, namely- podosomes (see Figure below). This “chapter” was based on a close collaboration with Prof. Lia Addadi.
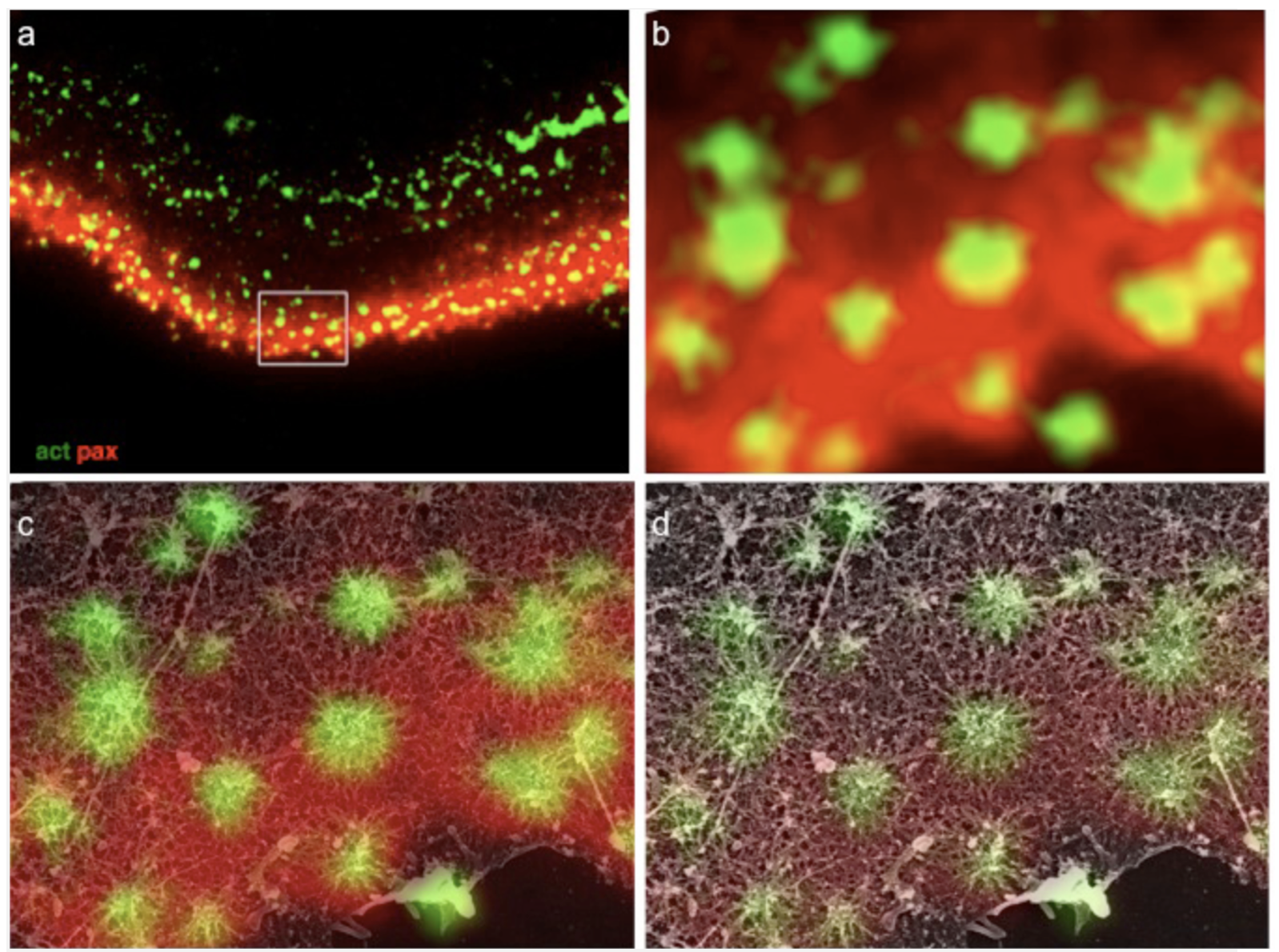
Structural relations between podosome ring and core domains. (a) Osteoclast ventral membranes were labeled for paxillin and actin, and simultaneously prepared for high-resolution scanning electron microscopy. (b) Higher magnification view of the area in the rectangle in (a). (c-d) Merged SEM and immunofluorescence images shows paxillin association with podosome radial actin fibers, reaching up to but not co-localizing with the central bundle.
Main findings:
- Characterization of the fine structure of podosomes using EM/LM-correlative microscopy
- Testing the effect of bone surface topography on osteoclast adhesion dynamics
- Spatial relationships between the “adhesion plaque” and
Osteoclastogenesis, osteoclast maturation, and the regulation of bone resorption
In a recent collaboration with Prof. Ari Elson, we have addressed a rare disease, called “osteopetrosis”, for deciphering basic mechanisms associated with the formation and maturation of osteoclasts (OCLs). These large, multi-nucleated cells are formed through well-regulated differentiation and cell fusion of monocyte-macrophage precursors. Disruption of OCL-mediated bone resorption perturbs bone production and homeostasis, which, in turn, can lead to serious illnesses, such as autosomal recessive osteopetrosis (ARO). Specifically, mutations in the intracellular trafficking-associated protein sorting nexin 10 (SNX10), lead to “OCL-rich” ARO, in which OCLs are produced but are inactive. Furthermore, cell fusion in SNX10-KO OCLs is deregulated, and mature mutant OCLs fuse continuously to generate gigantic cells, in vitro and in vivo, unlike wild-type OCLs that stop fusing upon maturation (see Figure below). In our studies, we explore the role of vesicular trafficking molecules and specific ion channels in regulating OCL fusion and bone resorption
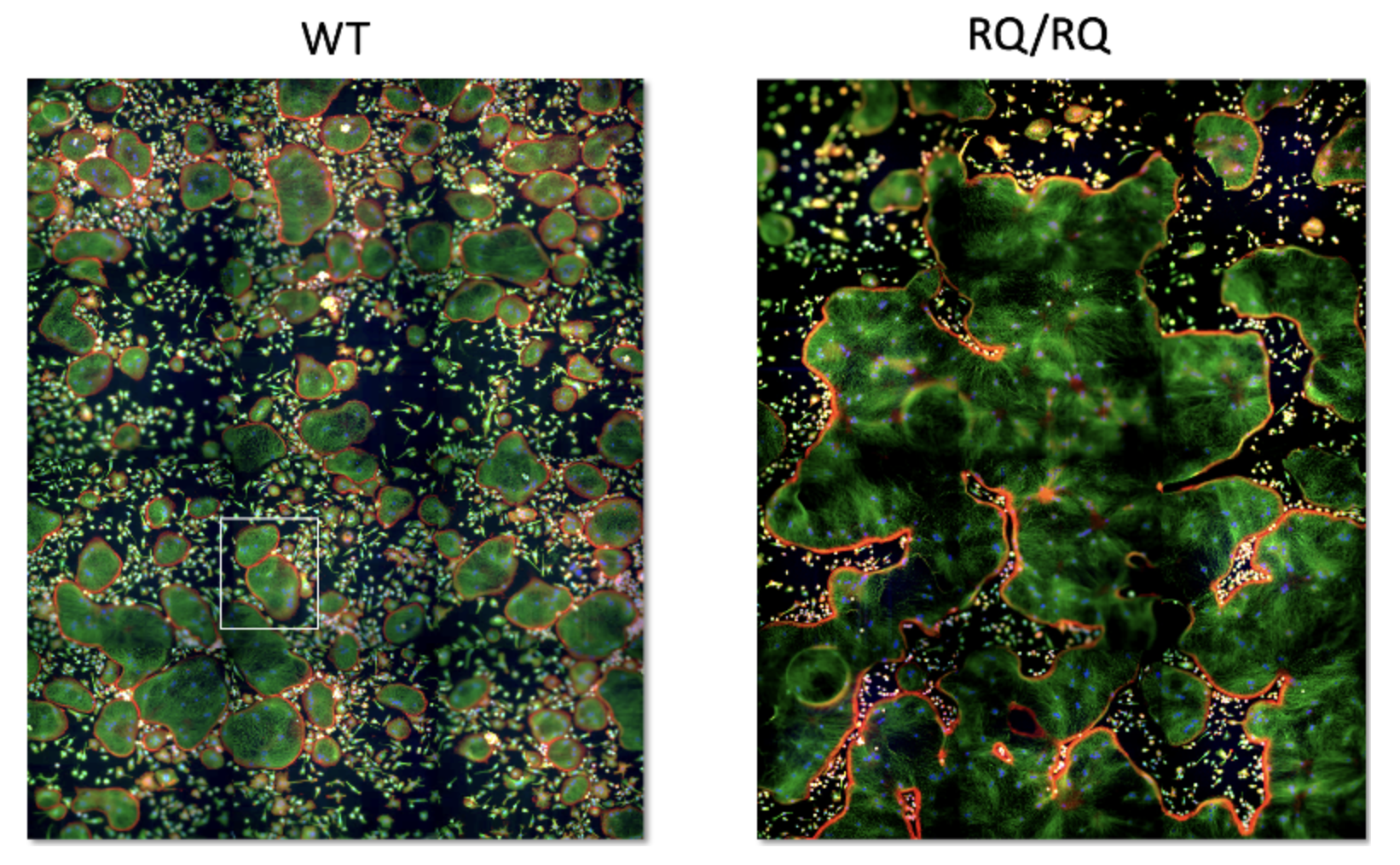
WT vs SNX10 giant mutant osteoclasts. Note that there are 2 WT osteoclasts in the square frame on the left, while the huge osteoclast on the right occupies nearly the entire image.
Main findings and achievements:
- Acquaintance with the genetic background of the R51Q mutation in SNX10 within the Palestinian population in the Hebron district of the West Bank
- Extensive documentation of the hyper-fusion phenotype
- Extensive documentation of the loss of resorption phenotype
- Discovery of similar phenotypes associated with other osteopetrotic mutations (OSTM1 and CLCN7)
- Involvement of SNX10 in endocytosis
Key collaborations: Lia Addadi, Ari Elson, Jan Tuckerman, Moien Kanaan, Leonid Chernomodrik
Selected Publications
- Elson A., Stein M., Rabie G., Barnea-Zohar M., Winograd-Katz S., Reuven N., Shalev M., Sekeres J., Kanaan M., Tuckermann J. and Geiger B. Sorting nexin 10 as a key regulator of membrane trafficking in bone- resorbing osteoclasts: Lessons learned from osteopetrosis. Front.Cell.Bio. 9:671210 (2021). doi: 10.3389/fcell.2021.671210.
- Barnea-Zohar M., Winograd-Katz S., Shalev M., Arman E., Roth L., Golani O., Stein S., Thalji F., Kanaan M., Tuckermann J., Geiger B., and Elson A. An SNX10-dependent mechanism down-regulates fusion between mature osteoclasts. J Cell Science. Jcs.254979 (2021). doi: 10.1242/jcs.254979
- Stein M., Barnea-Zohar M., Shalev M., Arman E., Brenner O., Winogrsd-Katz S., Gerstung J., Thalji F., Kanaan M., Elinav H., Stepensky P., Geiger B., Tuckermann J., Elson A. Massive osteopetrosis caused by non-functional osteoclasts in R51Q SNX10 mutant mice. Bone 136:115360 (2020). doi: 10.1016/j.bone.2020.115360.
- Barnea M., Stein M., Winograd-Katz S., Shalev M., Arman E., Brenner O., Thalji F., Kanaan M., Elinav H., Stepensky P., Geiger B., Tuckermann J., and Elson A. R51Q SNX10 induces osteopetrosis by promoting uncontrolled fusion of monocytes to form giant, non-functional osteoclasts. bioRxiv. doi: https://doi.org/10.1101/332551
- Levaot N., Ottolenghi A., Mann M., Kam Z., and Geiger B. Osteoclast fusion is initiated by a small subset of RANKL-stimulated monocyte progenitors, which can fuse to RANKL-unstimulated progenitors. Bone 79: 21-8 (2015) doi: 10.1016/j.bone.2015.05.021
- Luxenburg C, Winograd-Katz S, Addadi L and Geiger B. Involvement of actin polymerization in podosome dynamics. J Cell Sci 125(7): 1666-72 (2012)
- Geblinger D, Zink C, Spencer ND, Addadi L and Geiger B. Effects of surface micro-topography on the assembly of the osteoclast resorption apparatus. J Royal Soc Interface 9(72): 1599-608. https://doi.org/10.1098/rsif.2011.0659. (2012)
- Mann M, Barad O, Agami R, Geiger B and Hornstein E. miRNA-based mechanism for the commitment of multipotent progenitors to a single cellular fate. Proc Natl Acad Sci U S A 107 (36): 15804-9 (2010)
- Geblinger D, Addadi L and Geiger B. Nano-topography sensing by osteoclasts. J Cell Sci 123: 1503-10 (2010)
- Luxenburg C, Geblinger D, Klein E, Andersen K, Hanein D, Geiger B and Addadi L. The architecture of the adhesive apparatus of cultured osteoclasts: from podosome formation to sealing-zone assembly. PloS ONE. 2(1): e179 (2007)
