Cancer & Invasion
Invadopodia are actin-based protrusions of the plasma membrane that penetrate the extracellular matrix (ECM) and enzymatically degrade it. Invadopodia and podosomes, often referred to collectively as “endosomes,” are actin-based membrane protrusions that facilitate matrix remodeling and cell invasion across tissues, processes that occur under specific physiological conditions such as bone remodeling (see more in the “Bone and Osteoclasts” section), as well as under pathological states such as cancer invasion and metastasis (see invadopodia morphology in Figure 1 below.
Work in the Geiger lab specifically focused on the functional architecture and mechanobiological properties of invadopodia in cancer cells. Particularly, we studied three functional domains of invadopodia responsible for the metalloproteinase-based degradation of the ECM, the cytoskeleton-based mechanical penetration into the matrix, and the integrin adhesion-based adhesion to the ECM Figure 2).
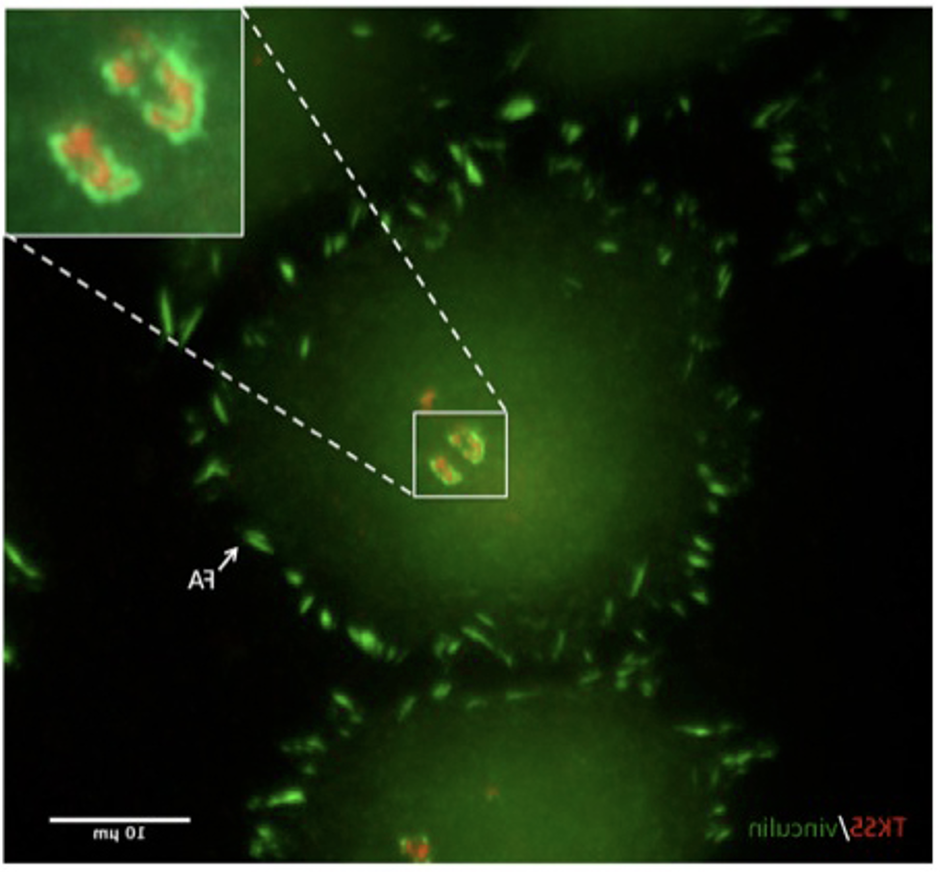
Figure 1: The actin core and adhesion ring of invadopodia: A375 cultured melanoma cells, growing on gelatin-coated glass-bottomed dishes, were fixed and stained for TKS5 (red), an invadopodia core marker, and vinculin (green), a marker for integrin adhesions. The adhesion ring (shown enlarged in the upper-right corner) is associated with the invadopodia core at early stages (within minutes) of invadopodia formation and often disappears at later stages (hours). Stable focal adhesions (FAs), located mainly at the cell’s periphery, are also formed in these cells.
The studies conducted at the Geiger laboratory included a novel observation, indicating that invadopodia tend to form “under’ the cells’ nuclei, with their core actin bundles “pushing against” the nucleus and indenting it. This observation, based on light-electron microscopy-based correlative imaging, supports a hypothesis that the nucleus might play a role in enhancing the mechanical penetration of invadopodia into the pericellular matrix. Further experiments demonstrated that the development of invadopodia is regulated by signaling cross-talk between different receptor tyrosine-kinases, and transcriptionally regulated by components of the Hippo pathway (YAP and TAZ).

Figure 2: Functional and structural domains of invadopodia, including the Adhesion Domain (driven by integrins, the proteolytic Domain, activated by receptor tyrosine kinases, and their target molecules, and the Invasive Domain consisting of actin and multiple actin regulators
Main Findings
- Assigning a mechano-regulatory role in invadopodia-invasion to the cell’s nucleus
- Highlighting similar mechano-regulatory mechanisms in invadopodia and focal adhesion that promote migration (FA-based) and invasion (invadopodia-based
- Discovery of cross-talk between ErbB and Axl kinases, associated with invadopodia formation
- Discovery of invadopodia-regulatory pathway, related to YAP/TAZ signaling, mediated via TKS5 and MMP14
Key collaborations: Moshe Oren, George Klein, Bentzi Katz, Werner Franke, Sasha Bershadsky
Selected Publications
- Gover-Proaktor., Leshem-Lev D., Winograd-Katz S., Partouche S., Samara A., Shapira S., Nardi-Agmon I., Harari E., Younis A., Najjar A., Kornowski R., Geiger B., Raanani P., Leader A., Granot G. Dasatinib induces endothelial dysfunction leading to impaired recovery from ischaemia. Br.J.Haematol. 205(3):1011-1016 (2024)
- Su M., Fleisher T., Grosheva I., Bokstad Horev M., Olszewska M., Mattioli Cioli C., Barr H., Plotnikov A., Caravalho S., Moskovitch Y., Minden MD., Chapal-Ilani N., Wainstein A., Papapetrou EP., Dezorella M., Cheng T., Kaushansky N., Geiger B., Shlush LI. Targeting SRSF2 Mutations in Leukemia with RKI-1447: A Strategy to Impair Cellular Division and Nuclear Structure. iScience. 27(4):109443 (2024)
- Balan Venghateri J., Dassa B., Morgenstern D., Shreberk-Shaked M., Oren M., Geiger B. Deciphering the involvement of the Hippo pathway co-regulators, YAP/TAZ in invadopodia formation and matrix degradation. Cell Death & Disease.14 (4): 290 (2023)
* Pre-publication in bioRxiv - Balan Venghateri J., Dassa B., Morgenstern D., Shreberk-Shaked M., Oren M., Geiger B. Deciphering the involvement of the Hippo pathway co-regulators, YAP/TAZ in invadopodia formation and matrix degradation. bioRxiv. doi: https://doi.org/10.1101/2022.06.28.497902 (2022)
- Su M., Fleisher T., Grosheva I., Bokstad-Horev M., Olszewska M., Barr H., Plotnikov A., Carvalho S., Moskovich Y., Minden M.D., Chapal-Ilani N., Papapetrou E.P., Dezorella N., Cheng T., Kaushansky N., Geiger B., Shlush L.I. Rock inhibitors target SRSF2 leukemia by disrupting cell mitosis and nuclear Morphology. bioRxiv. doi: https://doi.org/10.1101/2022.02.15.479934 (2022)
- Revach O-Y., Grosheva I., and Geiger B. Biomechanical regulation of focal adhesion and invadopodia formation. J Cell Science 133, jcs244848 (2020)
- Gohsh S., Nataraj N.B., Noronha A., Patkar S., Sekar A., Mukherjee S., Winograd-Katz S., Kramarski L., Verma A., Lindzen M., Garcia D.D., Green J., Eisenberg G., Gil-Henn H., Basu A., Lender Y., Weiss S., Oren M., Lotem M., Geiger B., Ruppin E., Yarden Y. PD-L1 recruits phospholipase C and enhances tumorigenicity of lung tumors harboring mutant forms of EGFR. Cell Reports 35(8):109181 (2021)
- Elisha Y, Sagi Y, Klein G, Straussman R and Geiger B. Cooperativity between stromal cytokines drives the invasive migration of human Breast cancer cells. The Royal Society 374:1779. (2019)
- Revach O-Y, Winograd-Katz SE, Samuels Y, and Geiger B. The involvement of mutant Rac1 in the formation of invadopodia in cultured melanoma cells. Exp Cell Res 343(1): 82-8 (2016).
- Revach O-Y, Weiner A, Rechav K, Sabanay I, Livne A, and Geiger B. Mechanical interplay between invadopodia and the nucleus in cultured cancer cells. Sci Rep 5: 9466 (2015).
- Van Roosmalen W, Le Dévédec SE, Golani O, Smid M, Timmermans AM, Look MP, Zi D, Pont C, de Graauw M, Naffar-Abu-Amara S, Kirsanova C, Rustici G,
Martens JWM, Foekens JA, Geiger B and van de Water B. Tumor cell migration screen identifies SRPK1 as breast cancer metastasis determinant. J Clin Inves 125(4): 1648-64 (2015). - Nadav-Dagan L, Shay T, Dezorella N, Naparstek E, Domany E, Katz B-Z, and Geiger B. Adhesive interactions regulate transcriptional diversity in malignant B cells. Mol Cancer Res 8(4): 482-93 (2010).
