Immunology
Cellular immunotherapy, a leading form of cancer treatment, enlists the “warriors” of our immune system, our T-cells, in the war on cancer. During preparations for the treatment, physicians take a sample of T-cells from the patient and activate them to make them divide rapidly and form a massive army of cancer-killing cells that are then injected back into the patient.
Even though there is huge potential in enlisting the immune system in the battle against cancer, the success rate for such treatments has so far been somewhat limited. One of the reasons is that after weeks of accelerated division, the “warrior T-cells” might be numerous, but they often become exhausted, and their killing powers dwindle. This limitation motivated Profs. Benny Geiger and Nir Friedman in the Immunology and Regenerative Biology Department to develop a new approach (the “synthetic immune niche” (SIN) treatment) that induces an increase in T-cell proliferation while maintaining or even enhancing the cells’ killing process.
Background:
Cell-cell and cell-matrix adhesions play key roles in the recruitment and activation of immune T-cells. Among the molecular adhesion components are integrins (β1 and β2), mediating the interactions of various immune cells to extracellular matrices and other immune cells, respectively. In our current attempts to create a synthetic “immune niche,” we employ diverse surface patterning approaches that might mimic “partnering cells”, which are regularly located in the immune niches, and replace them in the process of T-cell activation (Figure 1).
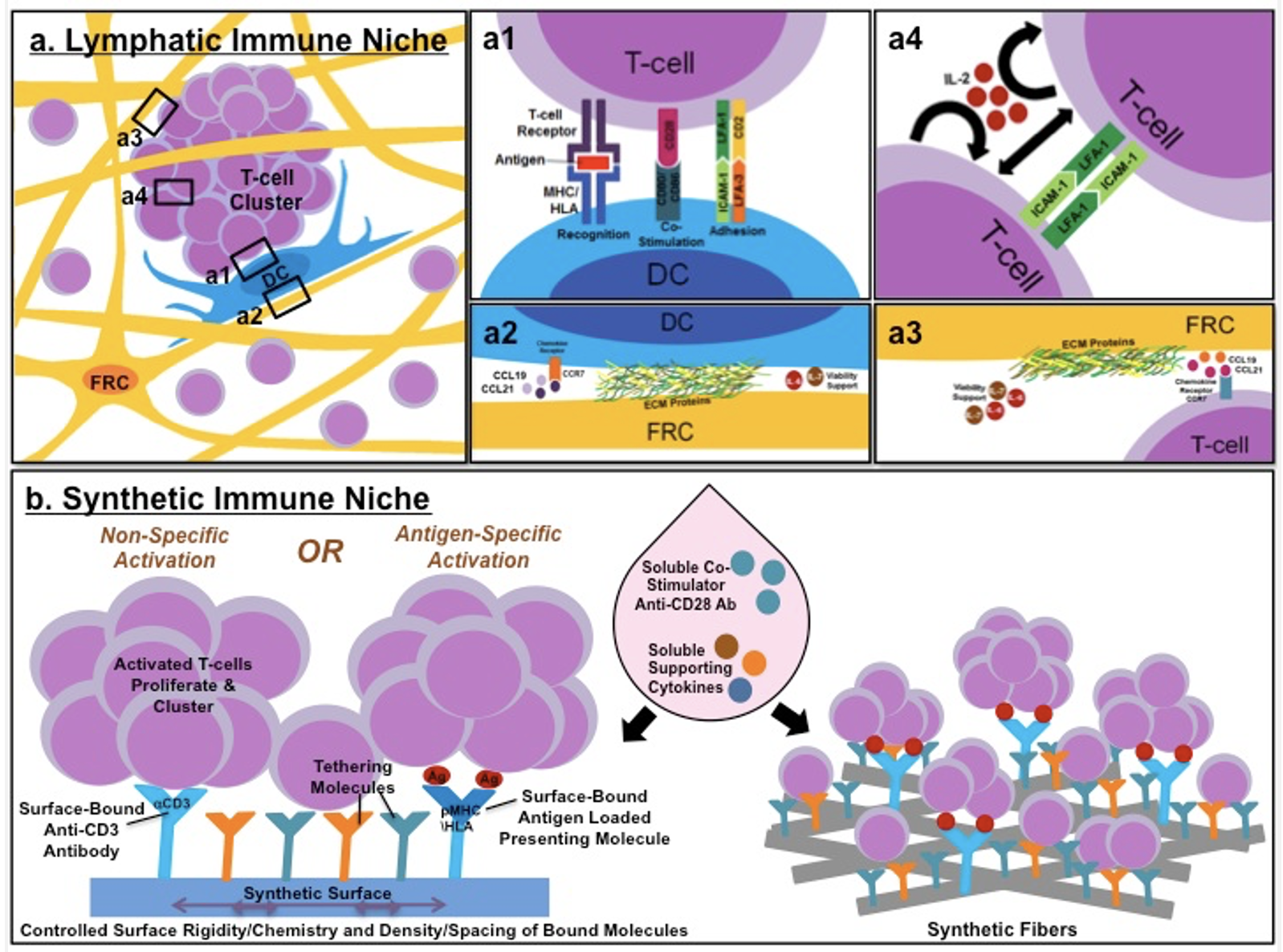
The complexity of the natural immune niches within lymph nodes. (a) Ly mph node-residing and infiltrating T-cells and dendritic cells (DC) migrate on a 3D network of fibroblastic reticular cells (FRCs) and their associated ECM. (a1) T cells can also interact with, and be activated by, DCs presenting via MHC/HLA a specific antigen matching the T-cell receptor, and accompanied by co-stimulatory (CD80/CD86 DCs, and CD28 T cells) and adhesive molecules (ICAM-1/LFA-3 DCs, and LFA-1/CD2 T cells). (a2,3) Both DCs and T cells interact with FRCs and their secreted chemokines (CCL19, CCL21), as well as cytokines (IL-6, IL-7), which promote their migration and survival. (a4) Activated T-cells form intercellular contacts with each other and cluster through ICAM-1-LFA-1 binding. Secretion of IL-2 by activated T-cells induces an autocrine- and paracrine-induced proliferative effect on adjacent T-cells. (b) In a novel approach for the construction of a “synthetic immune niche” (SIN), many of the cell-bound and ECM-associated ligands are transferred to a synthetic 2D (left) or 3D scaffold (right), to which T cells can bind. In the synthetic niche, interactions with the functionalized scaffold are expected to provide the signals needed for T-cell activation (with or without antigen specificity), lineage selection, proliferation, and survival.
The SIN technology:
In a series of studies, initiated together with Prof. Nir Friedman (who passed away on April 8, 2021) it was demonstrated that different classes of T-cells display an enhanced proliferation following treatment with immobilized CCL21 (a chemokine) and ICAM1(an adhesion molecule). It was further shown that this treatment enhanced the cancer-killing potential both ex vivo (in a petri dish) and in vivo (in live mouse models). In recent experiments, it was found that depending on the mode of activation of the T-cells, the SIN exerted its effect either by enhancing the killing machinery of the cells or by suppressing the exhaustion process.
Furthermore, while the initial experiments were performed primarily in mouse models, they were recently shown to be highly effective in enhancing the potency of human tumor-infiltrating lymphocytes. Toward these studies, an advanced quantitative imaging platform was developed.
Main findings and achievements:
- The SIN activity is based on the joint effect of CCL21 and ICAM1
- The effect is activation-mode specific (antigen-specific vs nonspecific)
- The SIN reaches maximal effect in specific “time windows
- The SIN activation leads to major transformations in cell morphology (size, polarity)
- An advanced imaging platform was developed, enabling the quantification of morphological and dynamic cellular features
- SIN transcriptional effects, affecting cell proliferation, activation, cytotoxicity, and exhaustion, were established
Key collaborations: Nir Friedman, Michal Besser
Selected Publications
- Yado S, Dassa B, Zoabi R, Reich-Zeliger S, Friedman N, Benjamin Geiger B. Molecular mechanisms underlying the modulation of T-cell proliferation and cytotoxicity by immobilized CCL21 and ICAM1. *Prepublication in BioRxiv (doi: https://doi.org/10.1101/2024.02.07.578152)
- Yado S, Dassa B, Zoabi R, Reich-Zeliger S, Friedman N, Benjamin Geiger B. Molecular mechanisms underlying the modulation of T-cell proliferation and cytotoxicity by immobilized CCL21 and ICAM1. Journal for ImmunoTherapy of Cancer (Submitted)
- Yunger S., Geiger B., Friedman N., Besser M.J., Adutler-Lieber S. Modulating the proliferative and cytotoxic properties of patient-derived TIL by a synthetic immune niche of immobilized CCL21 and ICAM1. Front.Oncol. 13:1116328 (2023)
* Pre-publication in bioRxiv. - Yunger S., Geiger B., Friedman N., Besser M.J., Adutler-Lieber S. Modulating the proliferative and cytotoxic properties of human TIL by a synthetic immune niche of immobilized CCL21 and ICAM1. BioRxiv. doi: https://doi.org/10.1101/2022.07.07.499105 (2022)
- Adutler-Lieber S, Friedman N, Geiger B. Expansion and anti-tumor cytotoxicity of T- cells are augmented by substrate-bound CCL21 and ICAM1. Front.Immunol 9:1303 (2018)
- Adutler-Lieber S, Zaretsky I, Sabanay H, Kartvelishvily W, Golani O, Geiger B and Friedman N. Substrate-bound CCL21 and ICAM1 combined with soluble IL-
6 collectively augment the expansion of antigen-specific murine CD4 T-cells. Blood adv. 1(15):1016-1030 (2017) - Adutler-Lieber S, Zaretsky I, Platzman I, Deeg J, Friedman N, Spatz JP, and Geiger B. Engineering of synthetic cellular microenvironments: Implications for immunity. J Autoimmunity 54:100-11 (2014)
