genes that regulate changes in cell adhesion and motility during cancer progression
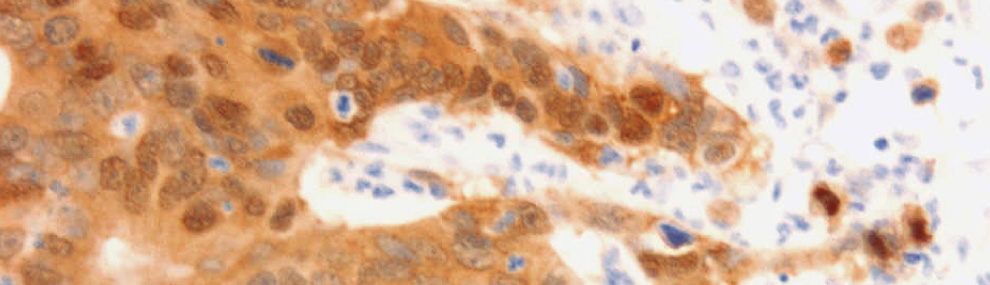
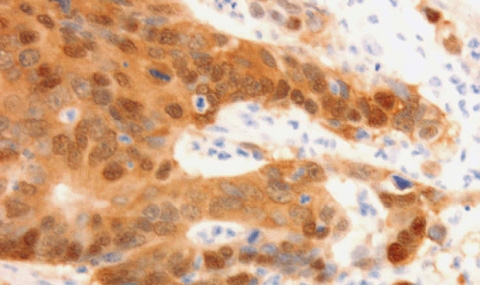
Our studies on Wnt/β-catenin target genes whose expression is aberrantly elevated during tumorigenesis identified L1 family members of transmembrane adhesion receptors, as targets of Wnt/β-catenin signaling. These receptors have critical functions in the brain by governing dynamic processes including axonal growth, fasciculation and pathfinding. Point mutations in L1 are associated with severe developmental brain diseases and mental retardation. How can the expression of L1 enhance the invasive potential of cancer cells? While L1 is categorized as a homophilic cell-cell adhesion receptor (like E-cadherin), there are profound differences in the properties of these receptors. While E-cadherin binds exclusively and very strongly to E-cadherin on the surface of adjacent cells, L1 binds only weakly to a variety of other molecules, including growth factor receptors, ECM components and integrins, in addition to L1 and other L1 family members. Thus, a cell adhesion receptor that can weakly bind to many extracellular ligands may constitute an ideal means for enhancing cancer cell motility when/if it replaces E-cadherin. Indeed, the increased expression of L1 in colon cancer cells (after Wnt/β-catenin activation) enhances cell motility, invasion, tumorigenesis and liver metastasis. Supporting this notion, in colon cancer tissue, L1 is exclusively expressed at the invasive edge of the tumor in cells displaying activated Wnt/β-catenin signaling (see figure below). We are investigating the mechanisms whereby L1 expression promotes cell motility and metastasis and the downstream genes affected when L1 expression is elevated.
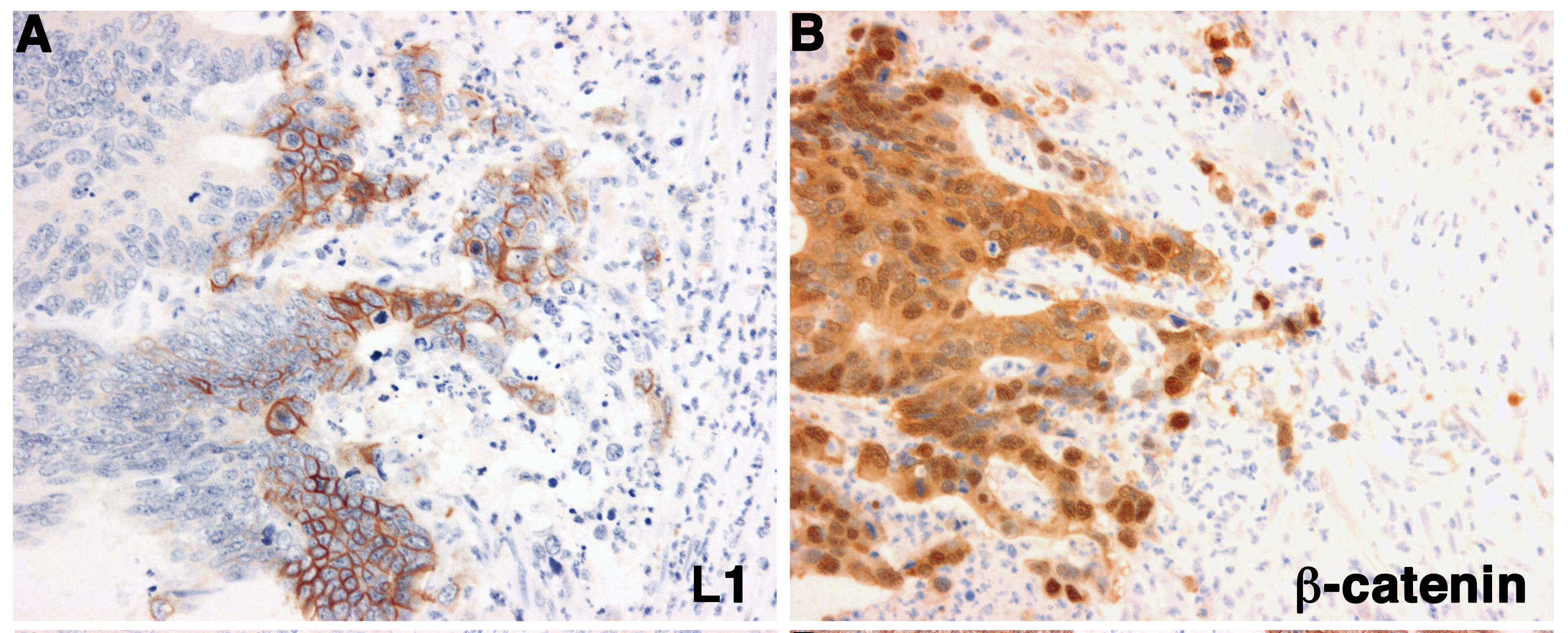
Human colorectal cancer (CRC) tissue displays strong β-catenin staining (including the nucleus), indicative of the activation of Wnt/β-catenin signaling in CRC cells (right panel). L1, a target gene of the Wnt/β-catenin pathway, is expressed exclusively in a subpopulation of CRC cells at the invasive edge of the tumor (left panel), pointing to its role in CRC invasion.