
Interaction of senescent cells with the immune system
Interaction of senescent cells with the immune system
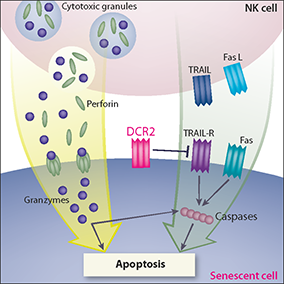 NK cells recognize and eliminate senescent cells to protect against liver fibrosis and to cause tumor regression after p53 reactivation or chemotherapy. To kill their target cells, NK cells use two mechanisms in parallel - granule exocytosis and activation of death receptors (Fig. 1). We recently discovered that inhibition of granule exocytosis or inhibition of granzyme activity prevents specific killing of the senescent cells by the NK cells in vitro (Sagiv et al., 2012). Moreover, in the mouse model of liver fibrosis, perforin knockout leads to more severe fibrosis and accumulation of senescent activated stellate cells in the liver. Therefore, perforin-mediated exocytosis is evidently necessary for NK-cell-mediated immune surveillance of senescent cells both in vitro and in vivo.
NK cells recognize and eliminate senescent cells to protect against liver fibrosis and to cause tumor regression after p53 reactivation or chemotherapy. To kill their target cells, NK cells use two mechanisms in parallel - granule exocytosis and activation of death receptors (Fig. 1). We recently discovered that inhibition of granule exocytosis or inhibition of granzyme activity prevents specific killing of the senescent cells by the NK cells in vitro (Sagiv et al., 2012). Moreover, in the mouse model of liver fibrosis, perforin knockout leads to more severe fibrosis and accumulation of senescent activated stellate cells in the liver. Therefore, perforin-mediated exocytosis is evidently necessary for NK-cell-mediated immune surveillance of senescent cells both in vitro and in vivo.
The mechanism that regulates the balance between killing of senescent cells by granule exocytosis and by death receptors is of particular interest. One reason why senescent cells are resistant to death induced by death-receptor ligands is upregulation of the decoy receptor DCR2 in senescent cells. This decoy receptor binds the death receptor ligands, preventing downstream signaling through competitive inhibition of the death-receptor pathway. Therefore, upregulation of DCR2 in senescent cells is responsible for the preferential killing of senescent cells through the granule exocytosis pathway (Fig. 1). High levels of DCR2 on the membranes of senescent cells also protect senescent cells from killing by soluble death-receptor ligands. This mechanism represents the first demonstration of regulatory machinery that allows specific killing of senescent cells.

