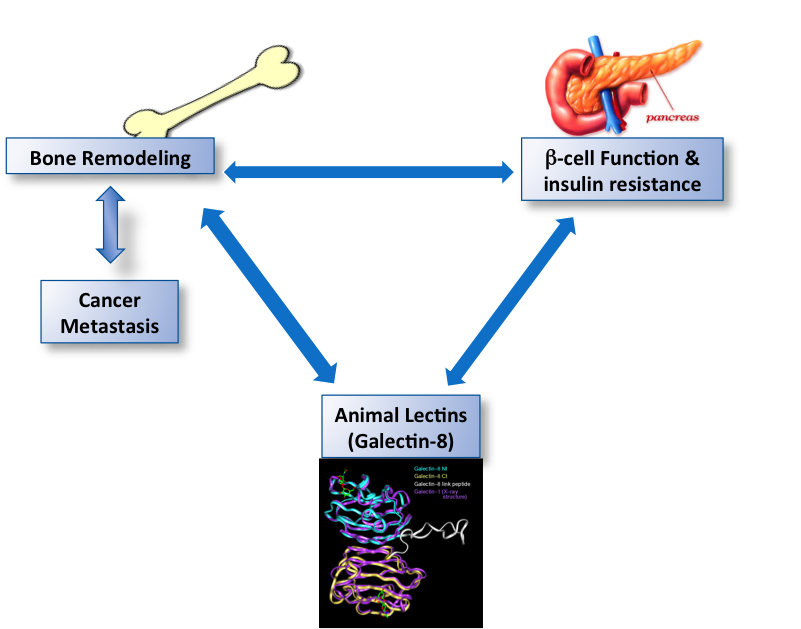
Galectins are a family of animal lectins, implicated in cell adhesion, cancer and immunity. We are specifically interested in galectin-8, originally cloned in our lab a number of years ago. Galectin-8 is an animal lectin which is secreted by an atypical secretory mechanism and binds to selected cell surface receptors. It regulates cell adhesion, growth and differentiation, as well as receptor trafficking. We have recently shown that galectin-8 triggers transcription of a unique set of genes, some of which are associated with bone remodeling. Skeletal integrity is maintained by the coordinated activity of osteoblasts, the bone forming cells and osteoclasts, the bone resorbing cells. We could show that mice overexpressing galectin-8 exhibit accelerated osteoclasts activity and bone turnover, which culminates in reduced bone mass, similar to cases of post-menopausal osteoporosis and cancerous osteolysis. This phenotype can be attributed to a direct action of galectin-8 on primary cultures of osteoblasts that secrete the osteoclastogenic factor RANKL upon binding of galectin-8. This results in enhanced differentiation into osteoclasts of bone-marrow cells co-cultured with galectin-8-treated osteoblasts. Secretion of RANKL by galectin-8-treated osteoblasts can be attributed to binding of galectin-8 to receptor complexes that positively (uPAR and MRC2) and negatively (LRP1) regulate galectin-8 function. Our findings identify galectins as new players in osteoclastogenesis and bone remodeling, and highlight a potential regulation of bone mass by animal lectins. Therefore, insights from this study might inform efforts to develop novel drug targets for the treatment of diseases associated with bone loss.