Publications
2026
-
(2026) Chemistry of Materials. 38, 2, p. 950-960 Abstract
Amorphous calcium carbonate phases are common intermediates in multistep crystallization processes. In many cases, it was shown that these dense and liquid-like phases function as transient metastable precursors that transform into mature crystalline phases. However, some biological systems consist of inorganic condensates that serve only as ion carriers and dissolve prior to the formation of the mineral. In this work, we study the chemical conditions that regulate the release of calcium ions from polymer condensates toward the formation of calcium carbonate. It is shown that the presence of bicarbonate ions tunes the stability of biogenic and bioinspired polymerCa condensates. In specific conditions of the carbonate system, condensate dissolution is induced, affecting calcium carbonate supersaturation and crystallization kinetics. This behavior recapitulates observations on the roles of such condensates in vivo, suggesting that bicarbonate ions indirectly affect mineralization by turning inorganic condensates from mineral precursors into sacrificial ion pools.
2025
-
(2025) Microscopy and Microanalysis. 31, 5, ozaf087. Abstract
Marine phytoplankton form functional biominerals with intricate morphologies and architectures. Coccolithophores occupy a special position among these organisms because of their production of intricate calcite scales, called coccoliths. Although coccolith morphologies differ across different species, crystals are organized around an organic matrix systematically to form an arrangement of astounding symmetry. We demonstrate the opportunities emerging from four-dimensional scanning transmission electron microscopy (4D-STEM), to spatially solve the crystallography of such biominerals. Through the development of a computational pipeline, which automatically solves the orientation at image pixels corresponding to crystals, we can map the orientation of the entangled and overlapping crystalline building blocks composing the coccolith. The present work exemplifies how parallel real space and diffraction space recordings can facilitate and improve the throughput of deciphering the complex network of biomineral superstructures.
(2025) Journal of Structural Biology. 217, 2, 108205. AbstractNanopatterning of inorganic materials is a challenging task for contemporary science. It is therefore remarkable that unicellular organisms can form intricately shaped biominerals. A prominent example is the silica cell wall of diatoms, which usually forms in specialized intracellular organelles. Inside such an organelle, biological regulation proceeds via the concerted activity of various organic macromolecules and inorganic precursors. However, it was shown that a specific type of elongated silica structures, called setae, which characterizes the diatom genus Chaetoceros, form extracellularly, raising questions about the regulatory mechanisms of this silicification process. Here, we study a relatively large species, Chaetoceros rostratus, that forms long and intricate setae. We used in-cell cryo electron tomography to image the native state of seta formation. The high-resolution 3D data show that silica formation outside the cell membrane involves continuous organic sheath that covers the newly formed seta. This sheath has an elaborate structure and is positioned tens of nanometers away from the silica by structural macromolecules that might be involved in architectural regulation. Elucidating the structural components of this delicate living system will allow for new opportunities to learn about the biological strategies for controlled mineralization.
(2025) Small. 21, 10, 2411965. AbstractTransient amorphous phases are known as functional precursors in the formation of crystalline materials, both in vivo and in vitro. A common route to regulate amorphous calcium carbonate (ACC) crystallization is via direct interactions with negatively charged macromolecules. However, a less explored phenomenon that can influence such systems is the electrostatically driven formation of Ca-macromolecule dense phases. In this study, it is shown how Ca-macromolecule condensates that form via liquidliquid phase separation (LLPS) can be used to control the formation of metastable ACC via diffusion-based mass transport. Contrary to the solid-like ACC particles that form in the dilute phase via rapid nucleation and growth, the condensate ACC gradually forms via carbonate diffusion into the dense droplets. This yields transient phases with non-stoichiometric compositions, similar to a solid solution. It is shown that the ability to control the concentration gradients across the phase boundary can be used to finely regulate the composition and stability of these amorphous precursors, offering new routes to control mineralization through transient phases.
(2025) Faraday Discussions. 261, p. 68-80 AbstractTo investigate the mechanisms of biomineral formation it is necessary to determine Ca concentrations in the vicinity of growing minerals at the highest spatial resolution. All techniques that use ionizing radiation will be limited by the onset of radiation damage. Limits to the minimum concentrations of Ca and minimum number of Ca atoms that can be detected are determined for EELS in TEM using the Ca L23 signal, and EDX in TEM using the Ca Kα line. A similar approach is used to set limits on what can be achieved via soft X-ray absorption spectroscopy detecting the Ca L23 features. Due to the large background, the lowest concentrations that can be detected via EELS are about 1 mM. Although the collection efficiency for EDX is inferior to that of EELS, the much lower background signal means that concentrations of about 0.05 mM could be detected, 20 times better than EELS. The spatial resolution of soft X-ray absorption spectroscopy is much lower, about 20 nm, but since the Ca L23 lines are at lower energies than the oxygen K edge that dominates the spectrum, the detection limits for Ca in an aqueous environment can be as low as 35 nM.
(2025) ISME Journal. 19, 1, wraf007. AbstractPelagic calcifying protists such as coccolithophores and foraminifera represent an important microbial component of the marine carbon cycle. Although their calcitic shells are preserved in oceanic sediments over millennia, their resilience in the future decades is uncertain. We review current literature describing the response of calcifying protists to ocean acidification and temperature warming. We examine these key ecological and biogeochemical processes through the cellular perspective, exploring the physiological, metabolic, and molecular responses of calcifying protists. Ocean acidification is a chemical process that takes place in the seawater outside the cell, whereas protists calcify inside a modified cellular microenvironment. The function of these calcification compartments depends on cellular response to ocean acidification, such as maintaining pH homeostasis. The response of calcifying protists to ocean acidification and temperature warming is species-specific, with no unifying trends but rather a range of sensitivity levels. Coccolithophores and foraminifera display physiological sensitivity that may hamper their ecological success in comparison to noncalcifying species. Yet, certain species may be more adaptable, especially when comparing to highly vulnerable calcifying molluscs as pteropods. As the molecular machinery mediating cellular calcification is not fully resolved, as well as the functional role of the calcitic shell, our ability to predict the fate of calcifying microorganisms in a warmer, more acidic ocean is limited. We propose the urgent need to expand the study of these model systems by advancing cell biology approaches and better understand the impact of climate change on microbial food webs in the ocean.
2024
-
(2024) ACS Nano. 18, 50, p. 33998-34006 Abstract[All authors]
Silica polymerization from its soluble monomers is fundamental to many chemical processes. Although industrial methods require harsh conditions and concentrated precursors, biological silica precipitation occurs under ambient conditions from dilute solutions. The hallmark of biosilica is the presence of amine-rich organic macromolecules, but their functional role remains elusive. Here, we show a pH-dependent stimulatory effect of such polyamines on silica polymerization. Notably, this process is decoupled from the saturation degree, allowing the synthesis of polymer-silica hybrid products with controlled network morphologies from undersaturated solutions. The data suggest a two-step phase separation process. First, an associative liquid-liquid phase separation forms a micrometer-size dense phase. Second, silica undergoes a liquid-to-solid transition in the supersaturated condensates to form a bicontinuous silica structure. This study can inspire \u201csoft chemistry\u201d routes to design organic-inorganic nanomaterials with regulatory principles optimized by evolution.
(2024) Advanced Functional Materials. 35, 7, 2415344. AbstractInorganic minerals that form via regulated biological processes exhibit remarkable properties. This is due to the involvement of macromolecules that control biomineralization. Even though the interactions of these biopolymers with solid mineral phases are intensely studied, not much is known about their involvement in the preceding steps of intracellular transport of the mineral building blocks. In this work, the model system of coccolith calcite crystallization is utilized to address the role of mineral-associated polysaccharides in the transport of calcium ions. State-of-the-art cryo-electron tomography is used to image in situ ion-rich dense phases in the wild-type and in two mutant strains, defected in coccolith production. The results show that the abundance and solubility of the calcium-rich condensates need to be finely tuned for proper crystallization. When the native macromolecular assemblage is compromised, calcium is still present in the calcifying fluid as a solute, but this is not sufficient for coccolith development. These results suggest that biomineralizing systems achieve superior regulation of crystallization due to the use of dense macromolecule-rich phases.
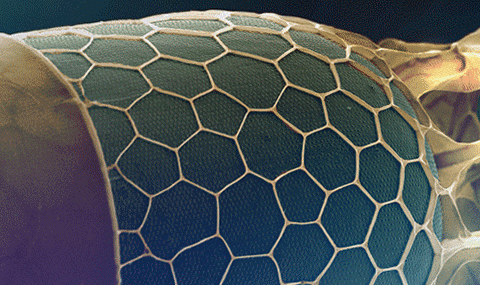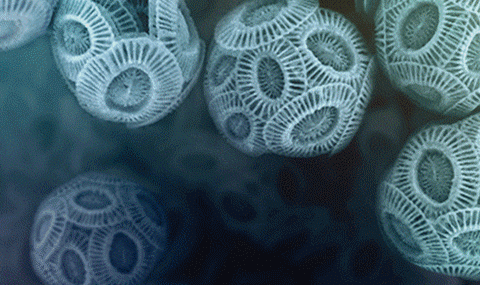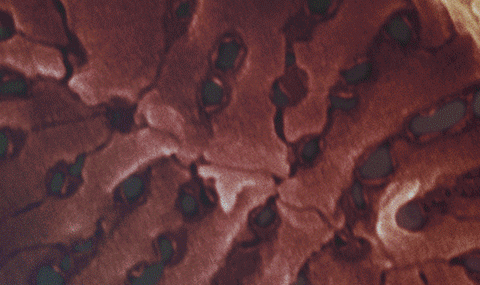
(2024) Advanced Science. 11, 41, 2402492. AbstractOrganisms are able to control material patterning down to the nanometer scale. This is exemplified by the intricate geometrical patterns of the silica cell wall of diatoms, a group of unicellular algae. Theoretical and modeling studies propose putative physical and chemical mechanisms to explain morphogenesis of diatom silica. Nevertheless, direct investigations of the underlying formation process are challenging because this process occurs within the confines of the living cell. Here, a method is developed for in situ 3D visualization of silica development in the diatom Stephanopyxis turris, using electron microscopy slice-and-view techniques. The formation of an isotropic hexagonal pattern made of nanoscale pores is documented. Surprisingly, these data reveal a directional process that starts with elongation of silica rods along one of the three equivalent orientations of the hexagonal lattice. Only as a secondary step, these rods are connected by crisscrossing bridges that give rise to the complete hexagonal pattern. These in situ observations combine two known properties of diatom silica, close packing of pores and branching of rods, to a unified process that yields isotropic patterns from an anisotropic background. Future research into diatom morphogenesis should focus on rod elongation and branching as the key for pattern formation.
(2024) Nature Communications. 15, 7888. Abstract[All authors]Silica cell-wall formation in diatoms is a showcase for the ability of organisms to control inorganic mineralization. The process of silicification by these unicellular algae is tightly regulated within a membrane-bound organelle, the silica deposition vesicle (SDV). Two opposing scenarios were proposed to explain the tight regulation of this intracellular process: a template-mediated process that relies on preformed scaffolds, or a template-independent self-assembly process. The present work points to a third scenario, where the SDV membrane is a dynamic mold that shapes the forming silica. We use in-cell cryo-electron tomography to visualize the silicification process in situ, in its native-state, and with a nanometer-scale resolution. This reveals that the plasma membrane interacts with the SDV membrane via physical tethering at membrane contact sites, where the curvature of the tethered side of the SDV membrane mirrors the intricate silica topography. We propose that silica growth and morphogenesis result from the biophysical properties of the SDV and plasma membranes.
Decoupling cell size homeostasis in diatoms from the geometrical constraints of the silica cell wall(2024) New Phytologist. 243, 1, p. 258-270 AbstractUnicellular organisms are known to exert tight control over their cell size. In the case of diatoms, abundant eukaryotic microalgae, two opposing notions are widely accepted. On the one hand, the rigid silica cell wall that forms inside the parental cell is thought to enforce geometrical reduction of the cell size. On the other hand, numerous exceptions cast doubt on the generality of this model. Here, we monitored clonal cultures of the diatom Stephanopyxis turris for up to 2 yr, recording the sizes of thousands of cells, in order to follow the distribution of cell sizes in the population. Our results show that S. turris cultures above a certain size threshold undergo a gradual size reduction, in accordance with the postulated geometrical driving force. However, once the cell size reaches a lower threshold, it fluctuates around a constant size using the inherent elasticity of cell wall elements. These results reconcile the disparate observations on cell size regulation in diatoms by showing two distinct behaviors, reduction and homeostasis. The geometrical size reduction is the dominant driving force for large cells, but smaller cells have the flexibility to re-adjust the size of their new cell walls.
(2024) Advanced Materials. 36, 11, 2309547. AbstractBiogenic crystals present a variety of complex morphologies that form with exquisite fidelity. In the case of the intricate morphologies of coccoliths, calcite crystals produced by marine algae, only a single set of crystallographic facets is utilized. It is unclear which growth process can merge this simple crystallographic habit with the species-specific architectures. Here, a suite of state-of-the-art electron microscopies is used to follow both the growth trajectories of the crystals ex situ, and the cellular environment in situ, in the species Emiliania huxleyi. It is shown that crystal growth alternates between a space filling and a skeletonized growth mode, where the crystals elongate via their stable crystallographic facets, but the final morphology is a manifestation of growth arrest. This process is reminiscent of the balance between reaction-limited and transport-limited growth regimes underlying snowflake formation. It is suggested that localized ion transport regulates the kinetic instabilities that are required for transport-limited growth, leading to reproducible morphologies.
2023
-
(2023) Nature Communications. 14, 1, 480. Abstract
Diatoms are unicellular algae characterized by silica cell walls. These silica elements are known to be formed intracellularly in membrane-bound silica deposition vesicles and exocytosed after completion. How diatoms maintain membrane homeostasis during the exocytosis of these large and rigid silica elements remains unknown. Here we study the membrane dynamics during cell wall formation and exocytosis in two model diatom species, using live-cell confocal microscopy, transmission electron microscopy and cryo-electron tomography. Our results show that during its formation, the mineral phase is in tight association with the silica deposition vesicle membranes, which form a precise mold of the delicate geometrical patterns. We find that during exocytosis, the distal silica deposition vesicle membrane and the plasma membrane gradually detach from the mineral and disintegrate in the extracellular space, without any noticeable endocytic retrieval or extracellular repurposing. We demonstrate that within the cell, the proximal silica deposition vesicle membrane becomes the new barrier between the cell and its environment, and assumes the role of a new plasma membrane. These results provide direct structural observations of diatom silica exocytosis, and point to an extraordinary mechanism in which membrane homeostasis is maintained by discarding, rather than recycling, significant membrane patches.
(2023) Small structures. 4, 7, 2200353. AbstractCoccolithophores are a group of unicellular marine algae that shape global geochemical cycles via the production of calcium carbonate crystals. Interestingly, different life-cycle phases of the same coccolithophore species produce very different calcitic scales, called coccoliths. In the widely studied diploid phase, the crystals have anisotropic and complex morphologies, while haploid cells produce coccoliths consisting solely of calcite crystals with simple rhombohedral morphology. Understanding how these two life-cycle phases control crystallization is a highly sought-after goal, yet, haploid phase crystallization has rarely been studied, and the process by which they form is unknown. Herein, advanced electron microscopy is employed to elucidate the cellular architecture of the calcification process in haploid cells. The results show that in contrast to diploid-phase calcification, the coccolith-forming vesicle of haploid-phase cells is voluminous. In this solution-like environment, the crystals nucleate and grow asynchronously in a process that resembles calcite growth in bulk solution, leading to the simple morphologies of the crystals. The two distinct mineralization regimes of coccolithophore life-cycle phases suggest that cellular architecture, and specifically confinement of the crystallization process, is a pivotal determinant of biomineral morphology and assembly.
(2023) NEW PHYTOLOGIST. 238, 1, p. 438-452 AbstractCRISPR/Cas enables targeted genome editing in many different plant and algal species including the model diatom Thalassiosira pseudonana. However, efficient gene targeting by homologous recombination (HR) to date is only reported for photosynthetic organisms in their haploid life-cycle phase. Here, a CRISPR/Cas construct, assembled using Golden Gate cloning, enabled highly efficient HR in a diploid photosynthetic organism.
Homologous recombination was induced in T. pseudonana using sequence-specific CRISPR/Cas, paired with a dsDNA donor matrix, generating substitution of the silacidin, nitrate reductase and urease genes by a resistance cassette (FCP:NAT).
Up to c. 85% of NAT-resistant T. pseudonana colonies screened positive for HR by nested PCR. Precise integration of FCP:NAT at each locus was confirmed using an inverse PCR approach. The knockout of the nitrate reductase and urease genes impacted growth on nitrate and urea, respectively, while the knockout of the silacidin gene in T. pseudonana caused a significant increase in cell size, confirming the role of this gene for cell-size regulation in centric diatoms.
Highly efficient gene targeting by HR makes T. pseudonana as genetically tractable as Nannochloropsis and Physcomitrella, hence rapidly advancing functional diatom biology, bionanotechnology and biotechnological applications targeted on harnessing the metabolic potential of diatoms.(2023) ACS biomaterials science & engineering. 9, 2, p. 601-607 AbstractMultistep mineralization processes are pivotal in the fabrication of functional materials and are often characterized by far from equilibrium conditions and high supersaturation. Interestingly, such 'nonclassical' mineralization pathways are widespread in biological systems, even though concentrating molecules well beyond their saturation level is incompatible with cellular homeostasis. Here, we show how polymer phase separation can facilitate bioinspired silica formation by passively concentrating the inorganic building blocks within the polymer dense phase. The high affinity of the dense phase to mobile silica precursors generates a diffusive flux against the concentration gradient, similar to dynamic equilibrium, and the resulting high supersaturation leads to precipitation of insoluble silica. Manipulating the chemistry of the dense phase allows to control the delicate interplay between polymer chemistry and silica precipitation. These results connect two phase transition phenomena, mineralization and coacervation, and offer a framework to achieve better control of mineral formation.
2022
-
(2022) Advanced Science. 9, 28, 2203444. Abstract[All authors]
Metal sulfides are a common group of extracellular bacterial biominerals. However, only a few cases of intracellular biomineralization are reported in this group, mostly limited to greigite (Fe3S4) in magnetotactic bacteria. Here, a previously unknown periplasmic biomineralization of copper sulfide produced by the magnetotactic bacterium Desulfamplus magnetovallimortis strain BW-1, a species known to mineralize greigite (Fe3S4) and magnetite (Fe3O4) in the cytoplasm is reported. BW-1 produces hundreds of spherical nanoparticles, composed of 12 nm substructures of a poorly crystalline hexagonal copper sulfide structure that remains in a thermodynamically unstable state. The particles appear to be surrounded by an organic matrix as found from staining and electron microscopy inspection. Differential proteomics suggests that periplasmic proteins, such as a DegP-like protein and a heavy metal-binding protein, could be involved in this biomineralization process. The unexpected periplasmic formation of copper sulfide nanoparticles in BW-1 reveals previously unknown possibilities for intracellular biomineralization that involves intriguing biological control and holds promise for biological metal recovery in times of copper shortage.
(2022) Acta Biomaterialia. 148, p. 336-344 AbstractBiomineralization processes exert varying levels of control over crystallization, ranging from poorly ordered polycrystalline arrays to intricately shaped single crystals. Coccoliths, calcified scales formed by unicellular algae, are a model for a highly controlled crystallization process. The coccolith crystals nucleate next to an organic oval structure that was termed the base plate, leading to the assumption that the base plate is responsible for the oriented nucleation of the crystals via stereochemical interactions. In recent years, several works focusing on a well-characterized model species demonstrated a fundamental role for indirect interactions that facilitate coccolith crystallization. Here, we develop the tools to extract the base plates from five different species, giving the opportunity to systematically explore the relations between base plate and coccolith properties. We used multiple imaging techniques to evaluate the structural and chemical features of the base plates under native hydrated conditions. The results show a wide range of properties, overlaid on a common rudimentary scaffold that lacks any detectable structural or chemical motifs that can explain direct nucleation control. This work emphasizes that it is the combination between the base plate and the chemical environment inside the cell that cooperatively facilitate the exquisite control over the crystallization process.
(2022) iScience. 25, 6, 104308. Abstract[All authors]In nature, bacteria reside in biofilms multicellular differentiated communities held together by an extracellular matrix. This work identified a novel subpopulationmineral-forming cellsthat is essential for biofilm formation in Bacillus subtilis biofilms. This subpopulation contains an intracellular calcium-accumulating niche, in which the formation of a calcium carbonate mineral is initiated. As the biofilm colony develops, this mineral grows in a controlled manner, forming a functional macrostructure that serves the entire community. Consistently, biofilm development is prevented by the inhibition of calcium uptake. Our results provide a clear demonstration of the orchestrated production of calcite exoskeleton, critical to morphogenesis in simple prokaryotes.
(2022) Angewandte Chemie - International Edition. 61, 17, e202115930. AbstractIn nature, simple organisms evolved mechanisms to form intricate biosilica nanostructures, far exceeding current synthetic manufacturing. Based on the properties of extracted biomacromolecules, polycation-polyanion pairs were suggested as moderators of biosilica formation. However, the chemical principles of this polymer-induced silicification remain unclear. Here, we used a biomimetic polycation-polyanion system to study polymer-induced silicification. We demonstrate that it is the polymer phase separation process, rather than silica-polymer interactions, which controls silica precipitation. Since ionic strength controls this electrostatic phase separation, it can be used to tune the morphology and structure of the precipitates. In situ cryo electron microscopy highlights the pivotal role of the hydrated polymer condensates in this process. These results pave the road for developing nanoscale morphologies of bioinspired silica based on the chemistry of liquid-liquid phase separation.
(2022) Science. 376, 6590, p. 312-316 AbstractDirecting crystal growth into complex morphologies is challenging, as crystals tend to adopt thermodynamically stable morphologies. However, many organisms form crystals with intricate morphologies, as exemplified by coccoliths, microscopic calcite crystal arrays produced by unicellular algae. The complex morphologies of the coccolith crystals were hypothesized to materialize from numerous crystallographic facets, stabilized by fine-tuned interactions between organic molecules and the growing crystals. Using electron tomography, we examined multiple stages of coccolith development in three dimensions. We found that the crystals express only one set of symmetry-related crystallographic facets, which grow differentially to yield highly anisotropic shapes. Morphological chirality arises from positioning the crystals along specific edges of these same facets. Our findings suggest that growth rate manipulations are sufficient to yield complex crystalline morphologies.
2021
-
(2021) Nature Communications. 12, 1, 4639. Abstract
Silica formation in diatoms is of interest for a range of different subjects from biomimetics to oceanography. Here the authors study the formation of silicified extensions in diatoms and find that unlike cell wall elements, that form in the cytoplasm, the extensions have a different formation mechanism outside the cytoplasm.
(2021) Journal of Structural Biology. 213, 4, 107807. AbstractUptake and concentration of inorganic ions are part of the complex cellular processes required for cell homeostasis, as well as for mineral formation by organisms. These ion transport mechanisms include distinct cellular compartments and chemical phases that play various roles in the physiology of organisms. Here, the prominent cases of dense ion pools in unicellular organisms are briefly reviewed. The specific observations that were reported for different organisms are consolidated into a wide perspective that emphasizes general traits. It is suggested that the intracellular ion pools can be divided into three types: a high cytoplasmic concentration, a labile storage compartment that hosts dense ion-rich phases, and a mineral-forming compartment in which a stable long-lived structure is formed. Recently, many labile pools were identified in various organisms using advanced techniques, bringing many new questions about their possible roles in the formation of the stable mineralized structures.
(2021) Journal of the American Chemical Society. 143, 50, p. 21100-21112 AbstractMinerals are formed by organisms in all of the kingdoms of life. Mineral formation pathways all involve uptake of ions from the environment, transport of ions by cells, sometimes temporary storage, and ultimately deposition in or outside of the cells. Even though the details of how all this is achieved vary enormously, all pathways need to respect both the chemical limitations of ion manipulation, as well as the many "housekeeping"roles of ions in cell functioning. Here we provide a chemical perspective on the biological pathways of biomineralization. Our approach is to compare and contrast the ion pathways involving calcium, phosphate, and carbonate in three very different organisms: the enormously abundant unicellular marine coccolithophores, the well investigated sea urchin larval model for single crystal formation, and the complex pathways used by vertebrates to form their bones. The comparison highlights both common and unique processes. Significantly, phosphate is involved in regulating calcium carbonate deposition and carbonate is involved in regulating calcium phosphate deposition. One often overlooked commonality is that, from uptake to deposition, the solutions involved are usually supersaturated. This therefore requires not only avoiding mineral deposition where it is not needed but also exploiting this saturated state to produce unstable mineral precursors that can be conveniently stored, redissolved, and manipulated into diverse shapes and upon deposition transformed into more ordered and hence often functional final deposits.
(2021) Proceedings of the National Academy of Sciences. 118, 46, e202567011. AbstractUnicellular marine microalgae are responsible for one of the largest carbon sinks on Earth. This is in part due to intracellular formation of calcium carbonate scales termed coccoliths. Traditionally, the influence of changing environmental conditions on this process has been estimated using poorly constrained analogies to crystallization mechanisms in bulk solution, yielding ambiguous predictions. Here, we elucidated the intracellular nanoscale environment of coccolith formation in the model species Pleurochrysis carterae using cryoelectron tomography. By visualizing cells at various stages of the crystallization process, we reconstructed a timeline of coccolith development. The three-dimensional data portray the native-state structural details of coccolith formation, uncovering the crystallization mechanism, and how it is spatially and temporally controlled. Most strikingly, the developing crystals are only tens of nanometers away from delimiting membranes, resulting in a highly confined volume for crystal growth. We calculate that the number of soluble ions that can be found in such a minute volume at any given time point is less than the number needed to allow the growth of a single atomic layer of the crystal and that the uptake of single protons can markedly affect nominal pH values. In such extreme confinement, the crystallization process is expected to depend primarily on the regulation of ion fluxes by the living cell, and nominal ion concentrations, such as pH, become the result, rather than a driver, of the crystallization process. These findings call for a new perspective on coccolith formation that does not rely exclusively on solution chemistry.
(2021) NEW PHYTOLOGIST. 231, 5, p. 1845-1857 Abstract[All authors]The development of calcification by the coccolithophores had a profound impact on ocean carbon cycling, but the evolutionary steps leading to the formation of these complex biomineralized structures are not clear. Heterococcoliths consisting of intricately shaped calcite crystals are formed intracellularly by the diploid life cycle phase. Holococcoliths consisting of simple rhombic crystals can be produced by the haploid life cycle stage but are thought to be formed extracellularly, representing an independent evolutionary origin of calcification. We use advanced microscopy techniques to determine the nature of coccolith formation and complex crystal formation in coccolithophore life cycle stages. We find that holococcoliths are formed in intracellular compartments in a similar manner to heterococcoliths. However, we show that silicon is not required for holococcolith formation and that the requirement for silicon in certain coccolithophore species relates specifically to the process of crystal morphogenesis in heterococcoliths. We therefore propose an evolutionary scheme in which the lower complexity holococcoliths represent an ancestral form of calcification in coccolithophores. The subsequent recruitment of a silicon-dependent mechanism for crystal morphogenesis in the diploid life cycle stage led to the emergence of the intricately shaped heterococcoliths, enabling the formation of the elaborate coccospheres that underpin the ecological success of coccolithophores.
(2021) Advanced Biology. 5, 6, 2000296. AbstractSome microorganisms, such as coccolithophores, produce an intricate exoskeleton made of inorganic solids. Coccoliths, the calcium carbonate scales of coccolithophores, are examples of the precise bioproduction of such complex 3D structures. However, the understanding of the cellular mechanisms that control mineral formation inside the cell, specifically the ability of these microalgae to transport high fluxes of inorganic building blocks, is still limited. Recently, using cryo-electron and X-ray microscopy, several intracellular compartments are shown to store high concentrations of calcium and phosphorous and are suggested to have a dominant role in the intracellular mineralization pathway. Here, live-cell confocal microscopy and fluorescent markers are used to examine the dynamics of ion stores in coccolithophores. Using calcein and 4,6-diamidino-2-phenylindole (DAPI) as fluorescent proxies for calcium and polyphosphates, the experiments reveal an unexpected plethora of organelles with distinct fluorescent signatures over a wide range of strains and conditions. Surprisingly, the fluorescent labeling does not show changes along the calcification process and is similar between calcifying and noncalcifying cells, suggesting that these ion pools may not be a dynamic avenue for calcium transport. In such a case, the enigma behind the ability of coccolithophores to sustain intracellular calcification still awaits comprehensive elucidation.
(2021) Chemistry of Materials. 33, 10, p. 3534-3542 AbstractMany organisms orchestrate the controlled precipitation of minerals. This physiological process takes place at ambient conditions, using soluble ions as building blocks. A widespread strategy for such crystallization processes is using a multistep route, where the initial phase is metastable and gradually transforms into the mature mineral phase. Even though the maturation of these intermediate phases has been intensively studied, it remains unclear how the initial, far from equilibrium phase can form within the cellular context. A model system for controlled biomineralization is the production of coccoliths by marine microalgae. Coccoliths are calcium carbonate crystalline arrays that form within the intracellular environment, at very low calcium concentrations. Here, we used coccolith-derived and synthetic polymers to study, in vitro, the chemical interactions between calcium ions and organic macromolecules that precede coccolith formation. We used in situ analyses, including state-of-the-art cryo-electron tomography and liquid-cell atomic force microscopy, to study the interactions in bulk solution and on organic surfaces simultaneously. The results unveil a chemical process in which a functional surface induces the precipitation of a polymerCa dense phase, or a coacervate, at chemical conditions where precipitation in solution is kinetically inhibited. This strategy demonstrates how organisms can form dense Ca-rich phases from the submillimolar concentration of calcium within organelles. This Ca-rich phase can then transform into a mineral precursor in a subsequent step, without posing challenges to cellular homeostasis.
2020
-
(2020) Science advances. 6, 42, 7554. Abstract
Diatoms are an abundant group of microalgae, known for their ability to form an intricate cell wall made of silica. Silicon levels in seawater are in the micromolar range, making it a challenge for diatoms to supply the rapid intracellular silicification process with the needed flux of soluble silicon. Here, we use three-dimensional cryoelectron microscopy and spectroscopy to quantitatively analyze, at submicrometer spatial resolution and sensitivity in the millimolar range, intracellular silicon in diatom cells. Our results show that the internal silicon concentration inside the cell is ~150 mM in average, three orders of magnitude higher than the external environment. The cellular silicon content is not compartmentalized, but rather unevenly distributed throughout the cell. Unexpectedly, under silicon starvation, the internal silicon pool is not depleted, reminiscent of a constitutive metabolite. Our spatially resolved approach to analyze intracellular silicon opens avenues to investigate this homeostatic trait of diatoms.
(2020) Journal of Structural Biology. 210, 1, 107465. AbstractThe formation of coccoliths, intricate calcium carbonate scales that cover the cells of unicellular marine microalgae, is a highly regulated biological process. For decades, scientists have tried to elucidate the cellular, chemical, and structural mechanisms that control the precise mineralogy and shape of the inorganic crystals. Transmission electron microscopy was pivotal in characterizing some of the organelles that orchestrate this process. However, due to the difficulties in preserving soluble inorganic phases during sample preparation, only recently, new intracellular ion-pools were detected using state-of-the-art cryo X-ray and electron microscopy techniques. Here, we combine a completely non-aqueous sample preparation procedure and room temperature electron microscopy, to investigate the presence, cellular location, and composition, of mineral phases inside mineral forming microalga species. This methodology, which fully preserves the forming coccoliths and the recently identified Ca-P-rich bodies, allowed us to identify a new class of ion-rich compartments that have complex internal structure. In addition, we show that when carefully choosing heavy metal stains, elemental analysis of the mineral phases can give accurate chemical signatures of the inorganic phases. Applying this approach to mineral forming microalgae will bridge the gap between the low-preservation power for inorganic phases of conventional chemical-fixation based electron microscopy, and the low-yield of advanced cryo techniques.
2019
-
(2019) CrystEngComm. 21, 23, p. 3586-3591 Abstract
As important optical devices, including diffuse scatterers, broadband reflectors, and image-forming mirrors with superior properties, biological guanine crystals have been thoroughly investigated in recent years. However, controlling the polymorph and morphology of synthetic guanine crystals is still highly challenging. Herein, a pure phase of the anhydrous guanine (AG) ss form was obtained via transformation of a hydrated amorphous guanine phase (HAmG) in solvents such as formamide, DMSO and DMF. The AG ss crystals can be stable for up to three months in the above solvents. The AG ss nano-platelets obtained in DMSO exposed the (100) plane when polyvinylpyrrolidone (PVP) was applied as an additive. The AG ss nano-platelets were about 100 nm in length, 40 nm in width, and 10-20 nm in thickness. Solid-state NMR (ssNMR) characterization indicated that the HAmG precursor had a similar short-range order as AG ss, which might be the reason for the formation of AG ss instead of the thermodynamically more stable AG a. The delicate control of the polymorph and morphology of the guanine crystals via an amorphous phase strategy may inspire the formation of highly ordered hierarchical structures of guanine crystals with unique optical properties.
(2019) Environmental and Experimental Botany. 157, p. 100-111 AbstractThe vegetative tissues of resurrection plants are able to withstand severe protoplasmic dehydration and revive quickly upon rehydration. Resurrection species defined as homoiochlorophyllous retain most or part of their chlorophyll and photosynthetic complement in the dry state, and rely on various mechanisms to protect themselves against photo-damage. In this study, we investigated the changes in chlorophyll distribution, light absorption gradients as well as the alterations in ultrastructure that take place during dehydration of the homoiochlorophyllous species Craterostigma pumilum. Chlorophyll fluorescence profiles show that light absorption is attenuated in dry leaves, likely minimizing generation of reactive oxygen species. These are accompanied by changes that take place in the supramolecular organization of the photosynthetic protein complexes, and ordered functional adjustments of the photosynthetic apparatus, further lessening the excitation and electron pressures. Albeit these, the ultrastructural studies reveal that chloroplasts in dehydrated leaf tissues exhibit features indicative of oxidative stress, which are also reminiscent of senescing chloroplasts. These include mass proliferation of plastoglobules, variable degrees of thylakoid dismantling, as well as chloroplast fragmentation and seemingly vacuolar degradation of such fragments. In addition, unique vesicular structures between the two chloroplast envelope membranes were visualized, some of which appeared to detach from chloroplasts, likely en route to degradation. Together, the data indicate that homoiochlorophyllous resurrection species handle photo-induced damage during dehydration on two levels. Minimization of photo-damage is achieved by attenuation of light absorption and other photo-protective mechanisms. When this is insufficient and significant damage does occur, elimination of damaged components takes place via processes resembling senescence. Nevertheless, these processes are reversible, enabling the plants to avoid the terminal steps of senescence and, hence, to recover.
2018
-
(2018) Proceedings of the National Academy of Sciences. 115, 43, p. 11000-11005 Abstract
Calcium storage organelles are common to all eukaryotic organisms and play a pivotal role in calcium signaling and cellular calcium homeostasis. In most organelles, the intraorganellar calcium concentrations rarely exceed micromolar levels. Acidic organelles called acidocalcisomes, which concentrate calcium into dense phases together with polyphosphates, are an exception. These organelles have been identified in diverse organisms, but, to date, only in cells that do not form calcium biominerals. Recently, a compartment storing molar levels of calcium together with phosphorous was discovered in an intracellularly calcifying alga, the coccolithophore Emiliania huxleyi, raising a possible connection between calcium storage organelles and calcite biomineralization. Here we used cryoimaging and cryospectroscopy techniques to investigate the anatomy and chemical composition of calcium storage organelles in their native state and at nanometer-scale resolution. We show that the dense calcium phase inside the calcium storage compartment of the calcifying coccolithophore Pleurochrysis carterae and the calcium phase stored in acidocalcisomes of the noncalcifying alga Chlamydomonas reinhardtii have common features. Our observations suggest that this strategy for concentrating calcium is a widespread trait and has been adapted for coccolith formation. The link we describe between acidocalcisomal calcium storage and calcium storage in coccolithophores implies that our physiological and molecular genetic understanding of acidocalcisomes could have relevance to the calcium pathway underlying coccolithophore calcification, offering a fresh entry point for mechanistic investigations on the adaptability of this process to changing oceanic conditions.
2017
-
(2017) Advanced Science. 4, 10, 1700088. Abstract
Many organisms form minerals from precursor phases that crystallize under strict biological control. The dynamic intracellular processes of formation, transport, and deposition of these precursor phases are challenging to identify. An unusual situation is recently revealed for the calcifying alga Emiliania huxleyi, as the cells contain a compartment filled with a concentrated Ca and P phase but the final calcite crystals, which are nucleated in a different compartment, are P-free. Thus, the connection of the Ca-P-rich pool to the mineralization process remains unclear. Here, pulse-chase experiments are used with Sr to label the Ca-P-rich phase in E. huxleyi cells, and cryo X-ray absorption spectroscopy and analytical transmission electron microscopy to follow the Sr within cells. It is found that Sr is first found in the Ca-P-rich phase and then becomes incorporated into the calcite. This demonstrates that the calcium used by the cells to build calcite originates from the Ca-P-rich pool.
(2017) Chemical Communications. 53, 55, p. 7740-7743 AbstractThe formation of intricately shaped crystalline minerals by organisms is orchestrated by specialized biomacromolecules. The macromolecules associated with coccoliths, nanometer-sized calcite crystal arrays produced by marine microalgae, can form a distinct calcium-rich phase via macromolecular recognition. Here, we show that this calcium-rich phase can be mineralized into a thin film of single-crystalline calcite by the balanced addition of carbonate ions. Such a crystallization process provides a strategy to direct crystalline products via local interactions between soluble macromolecules and compatible templates.
(2017) Advanced Materials Interfaces. 4, 1, 1600076. AbstractA liquidliquid phase boundary is experimentally determined in calcium carbonate solutions obtained by rapid mixing. Nanoparticles of amorphous calcium carbonate form at concentration higher than this boundary and their size diverges close to the boundary, which is interpreted as a spinodal line. At lower concentrations, the solution is metastable against the nucleation of crystalline calcium carbonate phases.
2016
-
(2016) Science. 353, 6299, p. 590-593 Abstract
Many organisms form elaborate mineralized structures, constituted of highly organized arrangements of crystals and organic macromolecules. The localization of crystals within these structures is presumably determined by the interaction of nucleating macromolecules with the mineral phase. Here we show that, preceding nucleation, a specific interaction between soluble organic molecules and an organic backbone structure directs mineral components to specific sites. This strategy underlies the formation of coccoliths, which are highly ordered arrangements of calcite crystals produced by marine microalgae. On combining the insoluble organic coccolith scaffold with coccolith-associated soluble macromolecules in vitro, we found a massive accretion of calcium ions at the sites where the crystals form in vivo. The in vitro process exhibits profound similarities to the initial stages of coccolith biogenesis in vivo.
(2016) Nature Communications. 7, 11228. Abstract[All authors]Coccoliths are calcitic particles produced inside the cells of unicellular marine algae known as coccolithophores. They are abundant components of sea-floor carbonates, and the stoichiometry of calcium to other elements in fossil coccoliths is widely used to infer past environmental conditions. Here we study cryo-preserved cells of the dominant coccolithophore Emiliania huxleyi using state-of-the-art nanoscale imaging and spectroscopy. We identify a compartment, distinct from the coccolith-producing compartment, filled with high concentrations of a disordered form of calcium. Co-localized with calcium are high concentrations of phosphorus and minor concentrations of other cations. The amounts of calcium stored in this reservoir seem to be dynamic and at a certain stage the compartment is in direct contact with the coccolith-producing vesicle, suggesting an active role in coccolith formation. Our findings provide insights into calcium accumulation in this important calcifying organism.
(2016) Israel Journal of Chemistry. 56, 4, p. 227-241 AbstractMany organisms build crystals with almost complete control over all aspects of crystal formation, from nucleation to growth, from composition to polymorphic structure, and from morphology to size. In biomineralization, the control is fundamentally always exerted at the level of nanometers, because the building blocks themselves are at the nanoscale. We have chosen to describe in some detail four biological systems that produce nanoscale crystals using different mineralization pathways, different levels of control, and have different functions. These four cases are: bone crystal composites; guanine nanocrystal reflectors; magnetotactic bacteria with single domain magnets; and coccoliths, whose functions have yet to be identified. This is followed by a discussion aimed at identifying possible specific and/or common underlying principles involved in nanocrystal formation in biology.
2015
-
(2015) CrystEngComm. 17, 13, p. 2606-2615 Abstract
Many organisms form crystals from transient amorphous precursor phases. In cases where the precursor phases were imaged, they were seen to consist of nanosphere particles. Interestingly, some mature biogenic crystals also have a nanosphere particle morphology, but some are characterized by crystallographic faces that are smooth at the nanometer level. There are also biogenic crystals that have both crystallographic faces and nanosphere particle morphology. This highlight presents a working hypothesis, stating that some biomineralization processes involve growth by nanosphere particle accretion, where amorphous nanoparticles are incorporated as such into growing crystals and their morphology is preserved upon crystallization. This process produces biogenic crystals with a nanosphere particle morphology. Other biomineralization processes proceed by ion-by-ion growth, and some cases of biological crystal growth involve both processes. We also identify several biomineralization processes which do not seem to fit this working hypothesis. It is our hope that this highlight will inspire studies that will shed more light on the underlying crystallization mechanisms in biology.
(2015) Journal of the American Chemical Society. 137, 2, p. 990-998 AbstractOrganisms tune the metastability of amorphous calcium carbonates (ACC), often by incorporation of additives such as phosphate ions and water molecules, to serve diverse functions, such as modulating the availability of calcium reserves or constructing complex skeletal scaffolds. Although the effect of additive distribution on ACC was noted for several biogenic and synthetic systems, the molecular mechanisms by which additives govern ACC stability are not well understood. By precipitating ACC in the presence of different PO43- concentrations and regulating the initial water content, we identify conditions yielding either kinetically locked or spontaneously transforming coprecipitates. Solid state NMR, supported by FTIR, XRD, and electron microscopy, define the interactions of phosphate and water within the initial amorphous matrix, showing that initially the coprecipitates are homogeneous molecular dispersions of structural water and phosphate in ACC, and a small fraction of P-rich phases. Monitoring the transformations of the homogeneous phase shows that PO43- and waters are extracted first, and they phase separate, leading to solid-solid transformation of ACC to calcite; small part of ACC forms vaterite that subsequently converts to calcite. The simultaneous water-PO43- extraction is the key for the subsequent water-mediated accumulation and crystallization of hydroxyapatite (HAp) and carbonated hydroxyapatite. The thermodynamic driving force for the transformations is calcite crystallization, yet it is gated by specific combinations of water-phosphate levels in the initial amorphous coprecipitates. The molecular details of the spontaneously transforming ACC and of the stabilized ACC modulated by phosphate and water at ambient conditions, provide insight into biogenic and biomimetic pathways.
"The price of freedom" - Factors affecting public support for the release of a captive soldier: The case of Gilad Shalit(2015) Economics Bulletin. 35, 4, p. 2184-2196 AbstractThis study examines the public support determinants for the release of terrorists in exchange for a single captive soldier. A sample of 751 Israelis were presented a questionnaire, in which the exchange price was manipulated into two versions: one in which no specific prices were mentioned, and another which presented a context-specific prisoner exchange. The results show that respondents who answered the non-specific version displayed greater support than those presented with specific details. Additionally, we found that soldiers tended to support the exchange more than civilians, and women showed greater support as compared to men. Our findings provide considerable support for the effect of framing and provide further evidence that decision-makers as well as media experts can guide public opinion.
2014
-
(2014) Journal of Applied Crystallography. 47, 5, p. 1651-1657 Abstract
Amorphous calcium carbonate phases, either synthesized artificially or generated biogenically, can be identified from broadened peaks in X-ray or electron diffraction profiles. It is conceivable that randomly oriented nanocrystals, approximately 1 nm in size, could give rise to coherent diffraction profiles that are characterized as amorphous. The coherent diffraction profiles for 200 keV electrons, as might be used in an electron microscope, and Cu Kα X-rays were calculated for needle-shaped calcite crystals bounded by {1121} facets and rhomb-shaped crystals bounded by {1014} facets. Crystals of about 1.0 nm in size gave a profile that is consistent with the X-ray measurements of amorphous calcium carbonate. The relative intensity of high-angle broadened peaks and changes in the IR spectrum are best explained by disorder in the nanocrystallites. The presence of randomly oriented nanocrystallites also explains the lack of optical birefringence.
(2014) Advanced Functional Materials. 24, 34, p. 5420-5426 AbstractMany biogenic minerals are composed of aggregated particles at the nanoscale. These minerals usually form through the transformation of amorphous precursors into single crystals inside a privileged space controlled by the organism. Here, in vitro experiments aimed at understanding the factors responsible for producing such single crystals with aggregated particle texture are presented. Crystallization is achieved by a two-step reaction in which amorphous calcium carbonate (ACC) is first precipitated and then transformed into calcite in small volumes of water and in the presence of additives. The additives used are gel-forming molecules, phosphate ions, and the organic extract from sea urchin embryonic spicules - all are present in various biogenic crystals that grow via the transformation of ACC. Remarkably, this procedure yields faceted single-crystals of calcite that maintain the nanoparticle texture. The crystals grow predominantly by the accretion of ACC nanoparticles, which subsequently crystallize. Gels and phosphate ions stabilize ACC via a different mechanism than sea urchin spicule macromolecules. It is concluded that the unique nanoparticle texture of biogenic minerals results from formation pathways that may differ from one another, but given the appropriate precursor and micro-environment, share a common particle accretion mechanism.
(2014) Journal Of Physical Chemistry B. 118, 28, p. 8449-8457 Abstract[All authors]X-ray absorption near-edge structure (XANES) spectroscopy and spectromicroscopy have been extensively used to characterize biominerals. Using either Ca or C spectra, unique information has been obtained regarding amorphous biominerals and nanocrystal orientations. Building on these results, we demonstrate that recording XANES spectra of calcium carbonate at the oxygen K-edge enables polarization-dependent imaging contrast (PIC) mapping with unprecedented contrast, signal-to-noise ratio, and magnification. O and Ca spectra are presented for six calcium carbonate minerals: aragonite, calcite, vaterite, monohydrocalcite, and both hydrated and anhydrous amorphous calcium carbonate. The crystalline minerals reveal excellent agreement of the extent and direction of polarization dependences in simulated and experimental XANES spectra due to X-ray linear dichroism. This effect is particularly strong for aragonite, calcite, and vaterite. In natural biominerals, oxygen PIC-mapping generated high-magnification maps of unprecedented clarity from nacre and prismatic structures and their interface in Mytilus californianus shells. These maps revealed blocky aragonite crystals at the nacre-prismatic boundary and the narrowest calcite needle-prisms. In the tunic spicules of Herdmania momus, O PIC-mapping revealed the size and arrangement of some of the largest vaterite single crystals known. O spectroscopy therefore enables the simultaneous measurement of chemical and orientational information in CaCO3 biominerals and is thus a powerful means for analyzing these and other complex materials. As described here, PIC-mapping and spectroscopy at the O K-edge are methods for gathering valuable data that can be carried out using spectromicroscopy beamlines at most synchrotrons without the expense of additional equipment.
2013
-
(2013) Angewandte Chemie (International ed.). 52, 18, p. 4867-4870 Abstract
An ugly duckling grows into a swan: Many organisms grow their crystalline mineral phases through the secondary nucleation of nanospheres made of an amorphous precursor phase. Stable amorphous calcium carbonate biominerals were used to induce a similar transformation in vitro. The amorphous nanospheres underwent a solidphase transformation that resulted in highly ordered calcite crystals composed of aggregated particles.
(2013) Journal of Cataract and Refractive Surgery. 39, 2, p. 292-294 AbstractAn 86-year-old patient developed a significant intraocular inflammatory reaction after having phacoemulsification. Topical therapy did not eliminate the inflammation, and tissue plasminogen activator (tPA) was injected into the anterior chamber. A white corneal plaque appeared in the previously clear cornea within days of the injection. The lesion was diagnosed as calcific band keratopathy and successfully treated with ethylenediaminetetraacetic acid chelation. Electron microscopy and elemental analysis of a corneal scraping from the lesion established its composition to be mainly calcium and phosphate, validating the diagnosis. This is the seventh reported case of rapid formation of calcific band keratopathy after tPA injection. The pathogenesis of this rare complication involves multiple factors, including alkalinization of the intraocular pH, increased phosphate concentration, and endothelial dysfunction. Financial Disclosure: No author has a financial or proprietary interest in any material or method mentioned.
2012
-
(2012) Chemistry-A European Journal. 18, 33, p. 10262-10270 Abstract
Plant cystoliths are mineralized objects that are formed by specialized cells in the leaves of certain plants. The main mineral component of cystoliths by volume is amorphous calcium carbonate (ACC) and the minor component is silica. We show that the silica stalk is formed first and is essential for ACC formation. Furthermore, the cystolith is shown to be composed of four distinct mineral phases with different chemical properties: an almost pure silica phase grades into a Mg-rich silica phase. This Mg-rich silica is overlaid by a relatively stable ACC phase. A bulky and less stable ACC phase encapsulates the first ACC phase. This architecture poses interesting questions about the role of Mg in the silica phase and suggests a strategy for ACC stabilization that takes advantage of a precise regulation of the mineral-growth microenvironment. The fantastic four: Cystoliths are mineralized objects that are mainly composed of amorphous calcium carbonate (ACC), which is found in the leaves of several plants. They have a unique composition and architecture of four distinct amorphous phases. A Mg-rich silica phase is essential for the formation of two distinct ACC phases. The inner ACC phase has inherently higher stability, presumably required by the sequential formation mechanism.
(2012) Advanced Materials. 24, 10, p. OP77-OP83 AbstractCystoliths are amorphous calcium carbonate bodies that form in the leaves of some plant families. Cystoliths are regularly distributed in the epidermis and protrude into the photosynthetic tissue, the mesophyll. The photosynthetic pigments generate a steep light gradient in the leaf. Under most illumination regimes the outer mesophyll is light saturated, thus the photosynthetic apparatus is kinetically unable to use the excess light for photochemistry. Here we use micro-scale modulated fluorometry to demonstrate that light scattered by the cystoliths is distributed from the photosynthetically inefficient upper tissue to the efficient, but light deprived, lower tissue. The results prove that the presence of light scatterers reduces the steep light gradient, thus enabling the leaf to use the incoming light flux more efficiently. MicroCT and electron microscopy confirm that the spatial distribution of the minerals is compatible with their optical function. During the study we encountered large calcium oxalate druses in the same anatomical location as the cystoliths. These druses proved to have similar light scattering functions as the cystoliths. This study shows that certain minerals in the leaves of different plants distribute the light flux more evenly inside the leaf. Leaf minerals function as internal light scatterers inside leaves. They transfer light from the saturated upper tissue into the light deprived lower tissue. This eases the steep light gradient inside the leaf and improves photosynthetic efficiency on the tissue scale.
2010
-
(2010) Journal of the American Chemical Society. 132, 38, p. 13208-13211 Abstract
Silicate ions increase the thermal stability of the unstable amorphous calcium carbonate (ACC). This effect was observed first by comparing ACC from two different species of cystoliths, small calcified bodies formed in the leaves of some plants. The temperature of crystallization to calcite in the silicate-rich cystoliths from M. alba is 100 °C higher than that of the silicate-poor cystoliths from F. microcarpa. The stabilizing effect is confirmed in vitro with synthetic samples differing in their silicate content. With increasing silicate concentration in ACC, the crystallization temperature to calcite also increases. A mechanism of geometric frustration is suggested, whereby the presence of the tetrahedral silicate ion in the flat carbonate lattice prevents organization into crystalline polymorphs.
2009
-
(2009) Environmental Chemistry. 6, 5, p. 416-423 Abstract
Environmental context. Since the 1960s the Dead Sea water level has dropped by nearly 30 m and over the last decade the rate of decline accelerated to over 1 m per year. Conveying seawater to the Dead Sea to stabilise or even raise its water level is currently being considered but may result in whitening of the surface water through the formation of minute gypsum crystals that will remain suspended in the water column for a prolonged period of time. This paper is a first step in attaining the relevant physical and chemical parameters required to assess the potential for such whitening of the Dead Sea.Abstract. Introduction of seawater to the Dead Sea (DS) to stabilise its level raises paramount environmental questions. A major concern is that massive nucleation and growth of minute gypsum crystals will occur as a result of mixing between the SO42-rich Red Sea (RS) water and Ca2+-rich DS brine. If the gypsum will not settle quickly to the bottom it may influence the general appearance of the DS by whitening the surface water. Experimental observations and theoretical calculations of degrees of saturation with respect to gypsum (DSG) and gypsum precipitation potentials (PPT) were found to agree well, over the large range but overall high ionic strength of DSRS mixtures. The dependency of both DSG and PPT on temperature was examined as well. Based on our thermodynamic insights, slow discharge of seawater to the DS will result in a relatively saline upper water column which will lead to enhanced gypsum precipitation.
-
-