Directed Evolution and Synthetic Biology
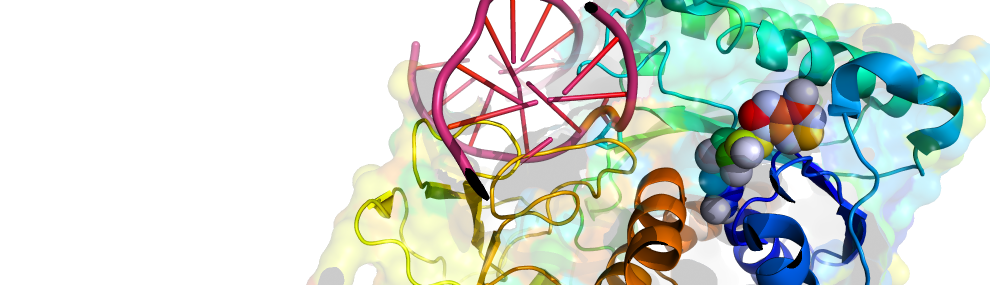
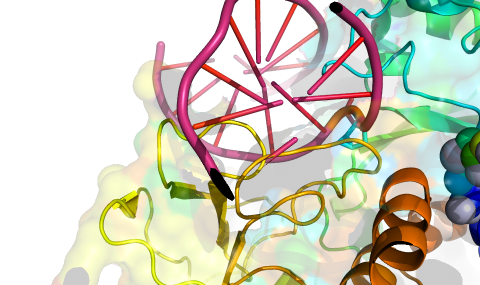
We apply state-of-the art approaches to evolve new enzymes and metabolic pathways. For example, enzyme systems can efficiently degrade and thus prevent intoxication by the most toxic nerve agent, VX. New metabolic pathways have been used to improve carbon fixation for eventual implementation in plants. Engineering methods include computational design, ‘smart’ library designs and high-throughput selection strategies.