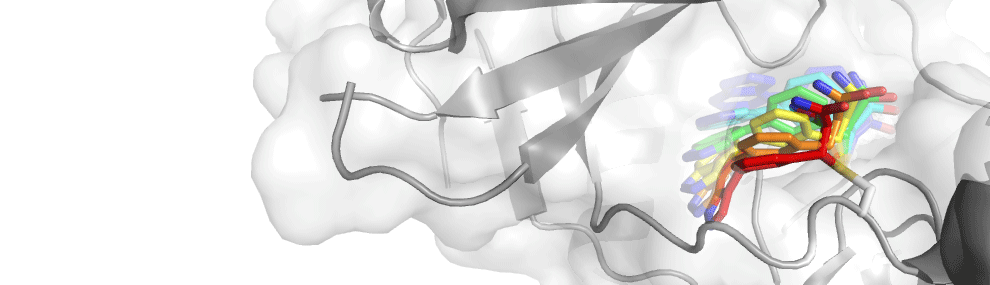
Allosteric binding sites are usually harder to target by small molecules, then pockets that were evolved to bind a substrate. Combined with an overall poor understanding of allostery it is currently very difficult to discover new chemical probes that allosterically modulate a protein function. Such molecules can have significant benefits, such as the ability to act as an activator rather than as a competitive inhibitor. Covalent binding molecules can potentially harness the covalent bond energy to stabilize high-energy protein conformations. We combine sophisticated structure prediction protocols using the Rosetta macromolecular modeling suite, with covalent virtual screening using DOCKovalent to design such allosteric binders. One of the model systems in the lab to develop and test this protocol is the K-Ras oncogene. Structures of K-Ras exposed a newly discovered pocket that is formed in part by the flexible switch-2 loop that mediates protein-protein interactions with Ras. Stabilizing this loop in different conformations could allostericaly fix Ras in a specific signaling state, and thus modulate its function independently of its activation state.