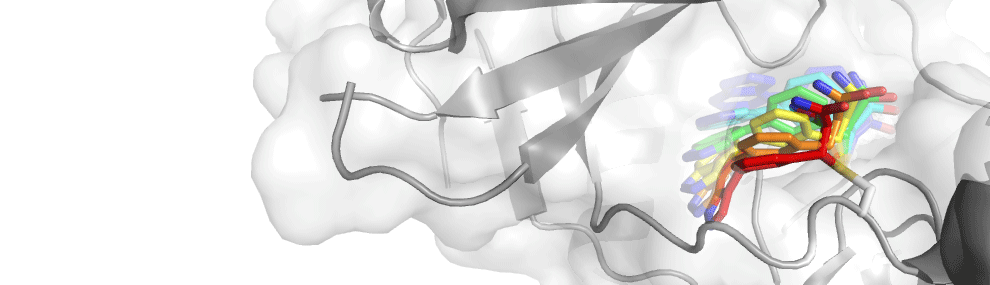
Kinase inhibitors have advantages over genetic approaches in elucidating kinase biology. Specific kinase inhibition however is challenging due to ATP binding-sites similarity. Covalent inhibitors targeting a unique active-site cysteine can overcome this challenge and often display remarkable specificity across the kinome.
In the past we were able to develop very specific reversible covalent inhibitors for two protein kinases RSK2 and JAK3. In the case of JAK3, targeting an active-site cysteine that is not found in any of the other JAK family members imparted exuisite selectivity on our compounds, with virtually no inhibition of JAK1, JAK2 and TYK2.
We are currently working on generalizing this approach to additional kinases, and forming a toolbox of specific covalent inhibitors for cysteine containing kinases.
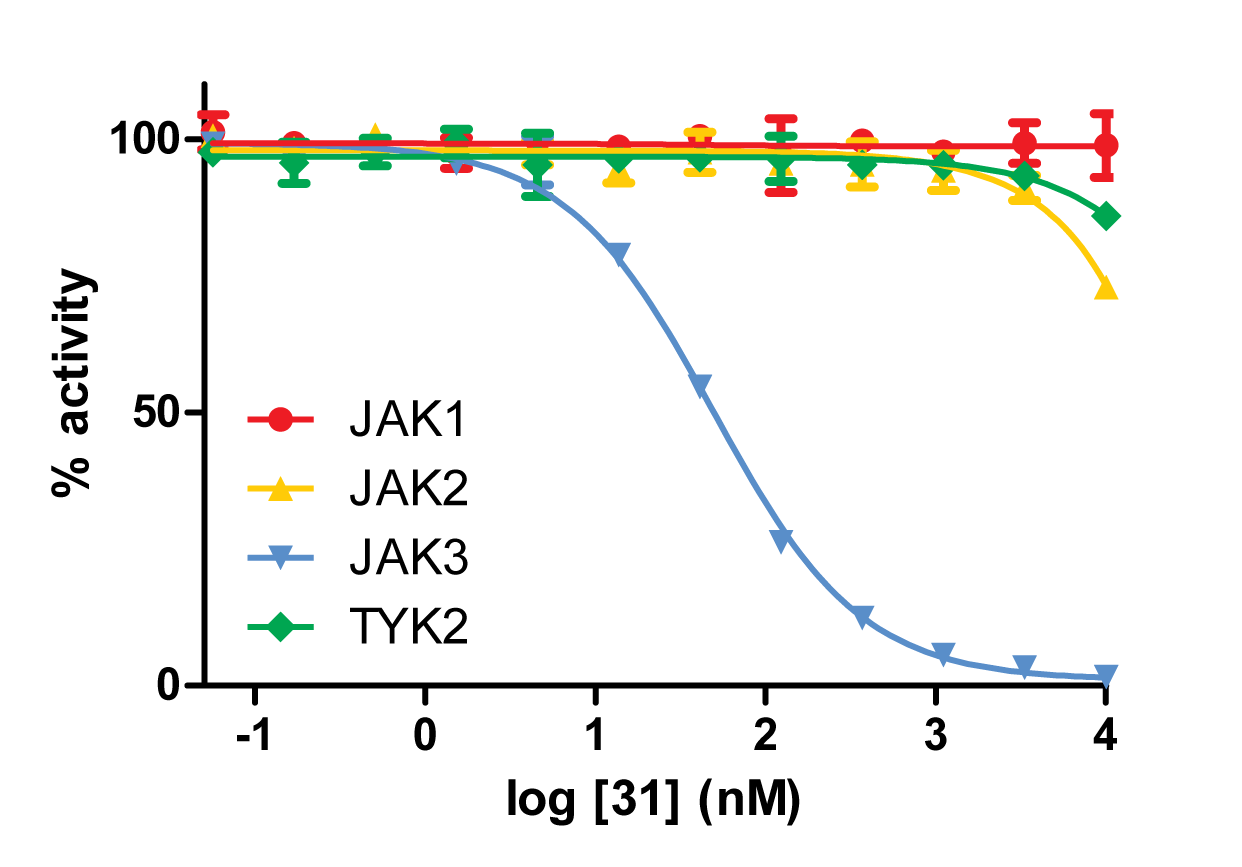
The best reversible covalent inhibitor discovered by docking for JAK3 (IC50=49nM) displayed marked selectivity against all other JAK kinase family members.