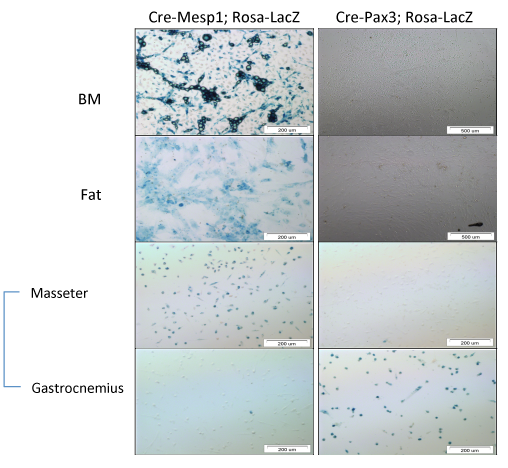
Mesenchymal stromal cells (MSCs) are progenitors, found in the bone marrow (BM) and in most if not all tissues. The ability of MSCs to differentiate into a variety of phenotypes and to proliferate in vitro, put them in the focus of stem cell research. In spite of the advances in the knowledge of MSC biology, there is little information regarding their natural distribution, the nature of their niche of residence and functions of MSCs in the living organism. In contrast, the in vitro properties of MSCs are well studied. In this study we aim at identifying the minimal culture requirements of un-manipulated MSCs and their embryonic origin. One way to enumerate MSC progenitors is to study in vitro colony formation by these cells, called colony-forming units-fibroblasts (CFU-Fs). Our results show that primary MSCs and nonadherent (NA)-MSCs, generated in vitro during the first week of culture, exhibit concentration dependence in CFU-F assays, contrary to cultured MSCs that are clonogenic in a concentration independent manner. Addition of mitogen stimulated spleen conditioned medium (S-CM) abolishes the concentration dependency of primary MSCs and NA-MSCs. Since the S-CM also elevates the incidence of macrophages in the cultures it is still unclear whether molecules in the S-CM directly affect CFU-Fs or that macrophages take part in this process. We intend to determine the in vitro growth requirements of CFU-Fs in order to get a clue as to the possible nature of their in vivo niche. Efforts are made by many laboratories to shed a light on the ontogeny and developmental origin of MSCs, however, to date little is known. Herein we are using a panel of Cre X loxP transgenic mice to trace the lineages of origin of adult MSCs. Our results demonstrate that BM and abdominal fat adult MSCs are in part from mesodermal Mesp1 origin and are not from ectodermal Pax3 origin. Furthermore, a portion of adult MSCs are from vascular endothelial (Tie2+ and VE-Cad+) origin. Currently we are extending the lineage tracing analysis to all three germ layers. In addition, we will compare the embryonic origin of MSCs from different adult tissues. Finally, modulation in MSC origin as a consequence of mouse age and tissue damage will be examined. The understanding of the in vivo biology of MSC is fundamental for the development of successful cell based therapies.