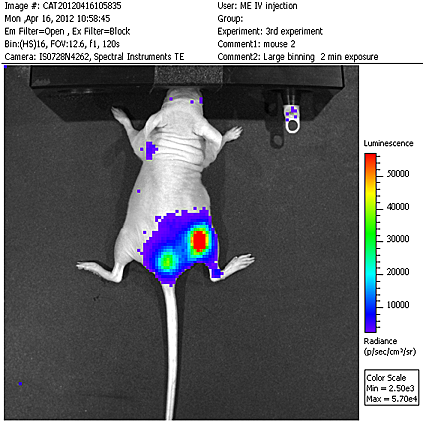
Human multiple myeloma (MM) is not effectively cured by current approaches, therefore, new therapeutic modalities for this cacner are called for. We found that activin A, a protein present in our body in minute amounts, kills MM cells, while sparing other blood cells. However, this protein also affects other organs and causes, for example, severe liver damage. It cannot therefore be use as a systemic drug. MM cancer cells specifically reside in the bone marrow compartment during early stages of the disease. Since mesenchymal cells create the microenvironment of the bone marrow and tend to localize there upon injection into the blood stream, we reasoned that we could use such cells to carry therapeutic molecules, such as activin A into the bone marrow specifically, preventing this protein drug from spreading to unwanted sites. In previous studies, we were able to show that injection of activin A sensitive mouse myeloma cells, simulates MM in mice. These cells predominantly migrate to the bone marrow, while only a residual accumulation is found in the spleen and liver. We were also able to show that mesenchymal cells isolated and propagated in culture, preferentially migrate into the bone marrow following injected into mice. We are currently preparing mesenchyme which produce high amouts of activin A, using molecular genetics methods. In the next stage, these cells will be used to determine the feasibility of causing regression of myeloma disease in mice. The proposed work is directed at finding a new therapeutic modality for the cure of multiple myeloma, by the use of cells, which co-localize with the myeloma cells in the inflicted bone marrow. Contrary to most currently used therapy modalities the proposed one is a cell and gene therapy approach. Our research group is maintains a close collaboration with the Hematology and Bone Marrow Transplantation Department in Sheba Medical Center with the aim of using the preclinical models studied in our laboratory for the planning of future clinical trials.