From biomass to biofuel
During the past several years, there has been increasing interest in the development of biofuels derived from environmentally friendly lignocellulosic biomass. The plant cell wall is composed mainly of cellulose, hemicellulose and lignin and is the most abundant carbon source on Earth. Therefore it represents a promising material for production of biofuels. One leading approach is to use consolidated bioprocessing (CBP), i.e., engineered microorganisms that are capable of directly converting lignocellulose into valuable end products (e.g., ethanol).
Lactobacillus plantarum is a common lactic acid bacterium that is used in variety of industrial and agriculture applications. This generally-recognized-as-safe (GRAS) bacterium is an attractive candidate for bioprocessing of lignocellulosic biomass to ethanol or higher alcohols, owing to is natural characteristics, which include high ethanol and acid tolerance and the ability to metabolize both pentose and hexose. However, it does not degrade cellulosic substrates nor does it produce ethanol or other possible types of biofuel.
One aim of our lab is to metabolically engineer L. plantarum to convert biomass into biofuels. In order to achieve this goal, we are working on introducing a variety of designer cellulosomes onto the surface of the L. plantarum cell. In addition, we are performing a series of deletion mutations and insertion of new genes into L. plantarum genome, in order to enhance ethanol production in L. plantarum. These efforts will potentially help us to convert L. plantarum into a consolidated bioprocessing (CBP) microorganism for producing high levels of alcohol directly from biomass.
Designer cellulosome assembly on L. plantarum cells
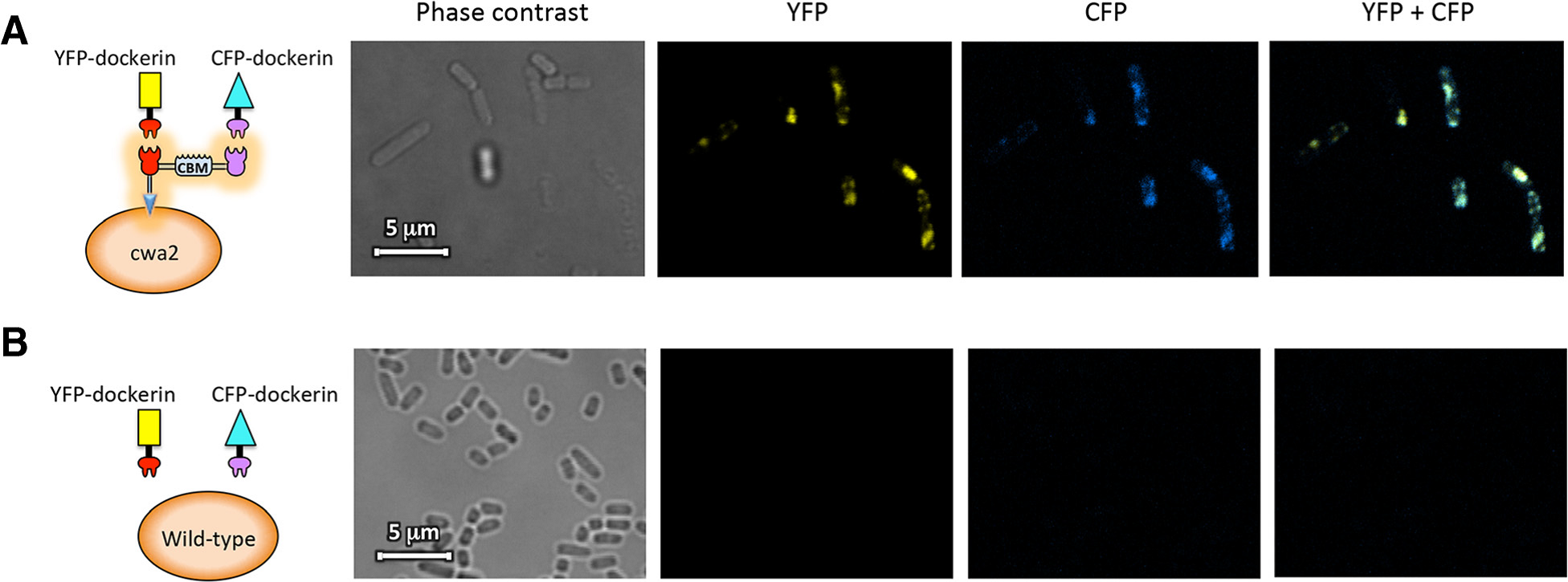
Confocal fluorescence microscopy images of scaffoldin Scaf·AT introduced into cells. The figure shows cells of a cwa2-carrying strain of L. plantarum (A) or wild-type cells (B) after binding interaction with mVenus yellow fluorescent protein (YFP) and mCerulean cyan fluorescent protein (CFP) fluorophores fused to the respective dockerin modules.
Functionality of the designer cellulosome paradigm
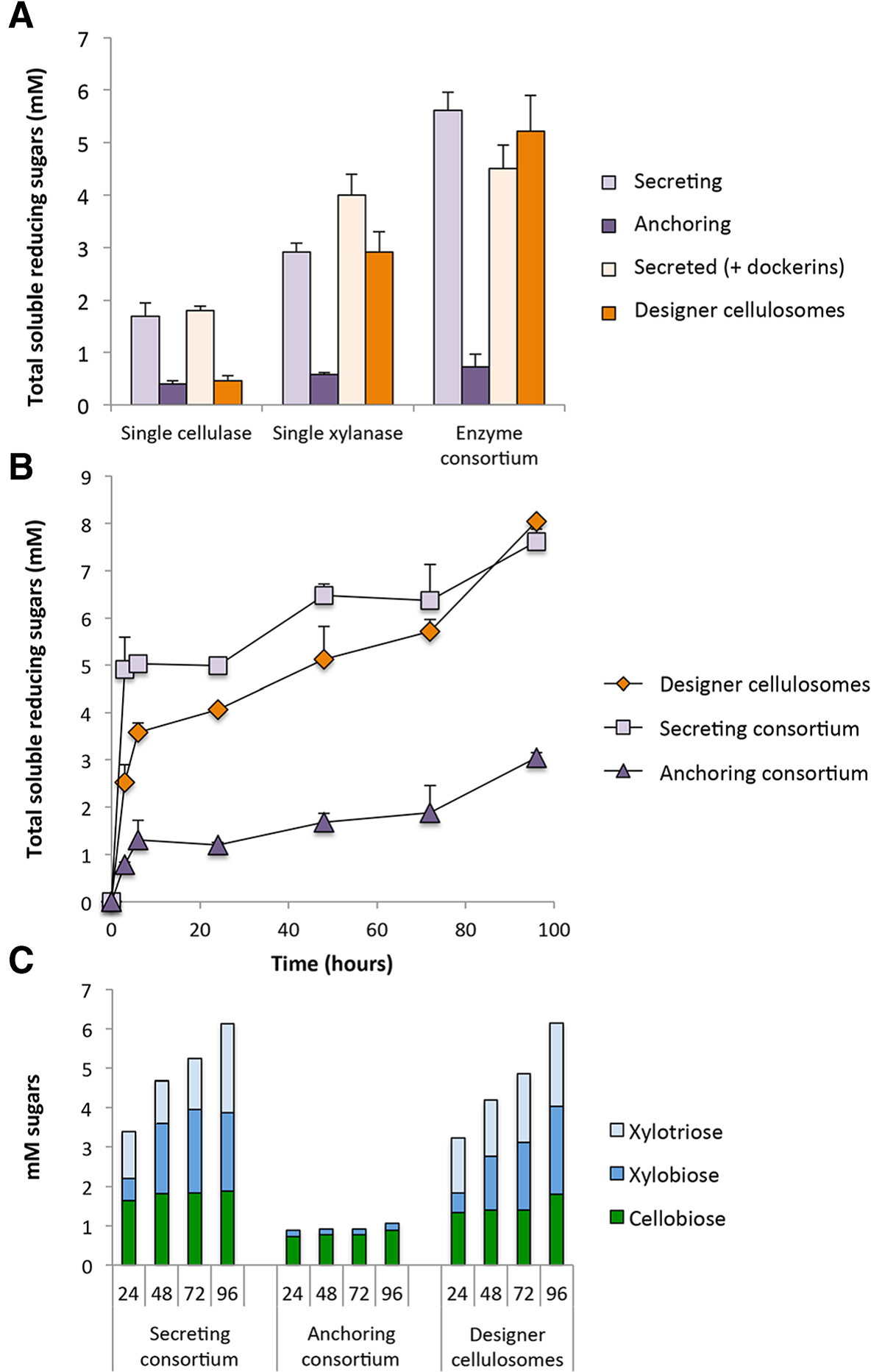
Enzyme activity studies. A. Comparative enzymatic activity of secreted or anchored cellulases and xylanases either individually or in a cell consortium. The concentration of hypochlorite pretreated wheat straw (dry matter) in the reactions was 40 g/l, and enzymes (either secreted or anchored) were added at 5 nM. Enzymatic activity is defined as mM soluble reducing sugars following a 24-hour reaction period at 37°C and pH 5. Each reaction was performed in triplicate and standard deviations are indicated. B. Kinetics studies of hypochlorite pretreated wheat straw hydrolysis by the secreted enzymatic consortium (squares) versus the designer cellulosome (diamonds) or the anchored enzymatic consortium (triangles). The concentration of hypochlorite pretreated wheat straw (dry matter) in the reactions was 40 g/l, and enzymatic consortium was added at 5 nM. Enzymatic activity is defined as mM soluble reducing sugars following a 3, 6, 24, 48, 72 or 96-hour reaction period at 37°C and pH 5. Each reaction was performed in triplicate and standard deviations are indicated. C. Soluble sugar production in mM as determined by HPLC analysis following digestion of hypochlorite retreated wheat straw over a 24, 48, 72 or 96-hour incubation period by the designated cell consortium. Glucose, xylose, arabinose and cellotriose were not detected.
Moraïs, S., Shterzer, N., Rozman Grinberg, I., Mathiesen, G., Eijsink, V. G. H., Axelsson, L., Lamed, R., Bayer, E. A., and Mizrahi, I. (2013) Establishment of a simple Lactobacillus plantarum cell consortium for cellulase-xylanase synergistic interactions. Appl. Environ. Microbiol. 79, 5242-5249.
Moräis, S., Shterzer, N., Lamed, R., Bayer, E. A., and Mizrahi, I. (2014) A combined cell-consortium approach for lignocellulose degradation by specialized Lactobacillus plantarum cells. Biotechnol. Biofuels 7:112.



