Cellular signaling requires messengers whose concentration varies with time and space. Ca2+ ions were selected early in evolution to play a major part in cellular signaling, and hundreds of proteins have adapted to bind Ca2+ over a million-fold range of affinities (nM to mM). Some of these proteins merely buffer local Ca2+ concentrations, while others may trigger complex cellular responses. The speed and effectiveness of Ca2+ signaling stems from a 20,000-fold gradient maintained by cells between their intracellular (100 nM free) and extracellular (mM) Ca2+ concentrations. The opening of selective Ca2+ channels at the plasma membrane triggers rapid inflow of Ca2+ ions, which are then sensed by Ca2+ binding proteins that tri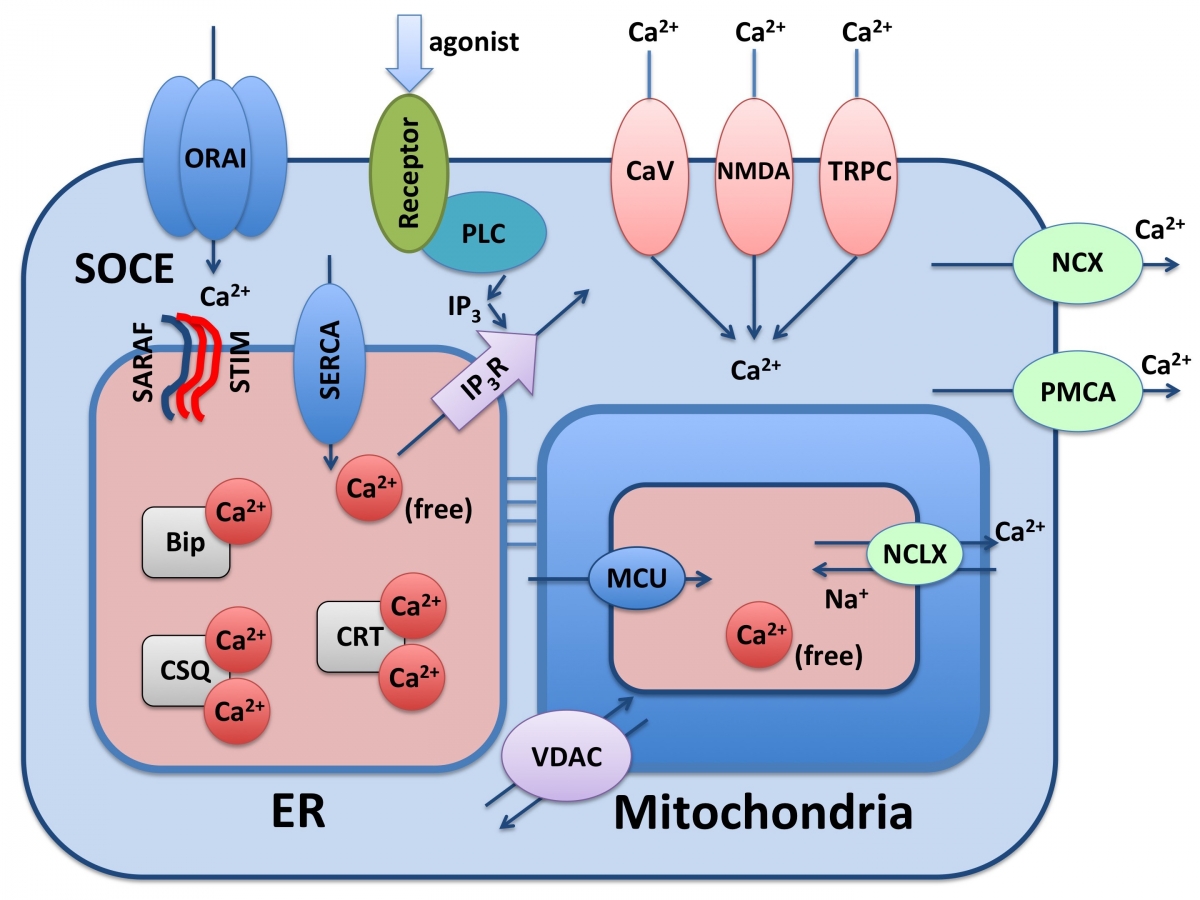 gger various cellular responses. An additional source for cellular Ca2+ signaling resides in organelles such as the mitochondria and the endoplasmic reticulum (ER) that have relatively high Ca2+ concentrations (hundreds of micromolars), and thus able to release Ca2+ ions into the cytoplasm. The amplitude, duration and kinetics of the Ca2+ transients mediated by these so-called intracellular Ca2+ stores affect diverse functions such as gene expression, proliferation, migration, cytokine release, salt balance and electrical activity. Store Operated Calcium Entry (SOCE) is the process that refills the cellular Ca2+ stores following Ca2+ discharge, and regulates basal cytoplasmic Ca2+. When the Ca2+ level in the ER lumen drops following Ca2+ signaling events, STIM, a single-pass ER membrane protein, senses the depleted calcium and by oligomerization and clustering into specialized plasma-membrane – ER membrane junctions activates ORAI - a selective plasma membrane Ca2+ channel. ORAI mediates Ca2+ influx into the cell to refill the ER via the Ca2+-ATPase pump, SERCA. This process, in which Ca2+ ions travel from the extracellular milieu (2mM) through the cell cytoplasm (100nM) and into the ER lumen (0.4mM), must be tightly regulated both temporally and spatially to prevent Ca2+ overfilling of the ER or spillover to the cytoplasm. We have recently identified SARAF (SOCE Associated Regulatory Factor), as a negative regulator of Ca2+ entry during SOCE.
gger various cellular responses. An additional source for cellular Ca2+ signaling resides in organelles such as the mitochondria and the endoplasmic reticulum (ER) that have relatively high Ca2+ concentrations (hundreds of micromolars), and thus able to release Ca2+ ions into the cytoplasm. The amplitude, duration and kinetics of the Ca2+ transients mediated by these so-called intracellular Ca2+ stores affect diverse functions such as gene expression, proliferation, migration, cytokine release, salt balance and electrical activity. Store Operated Calcium Entry (SOCE) is the process that refills the cellular Ca2+ stores following Ca2+ discharge, and regulates basal cytoplasmic Ca2+. When the Ca2+ level in the ER lumen drops following Ca2+ signaling events, STIM, a single-pass ER membrane protein, senses the depleted calcium and by oligomerization and clustering into specialized plasma-membrane – ER membrane junctions activates ORAI - a selective plasma membrane Ca2+ channel. ORAI mediates Ca2+ influx into the cell to refill the ER via the Ca2+-ATPase pump, SERCA. This process, in which Ca2+ ions travel from the extracellular milieu (2mM) through the cell cytoplasm (100nM) and into the ER lumen (0.4mM), must be tightly regulated both temporally and spatially to prevent Ca2+ overfilling of the ER or spillover to the cytoplasm. We have recently identified SARAF (SOCE Associated Regulatory Factor), as a negative regulator of Ca2+ entry during SOCE.
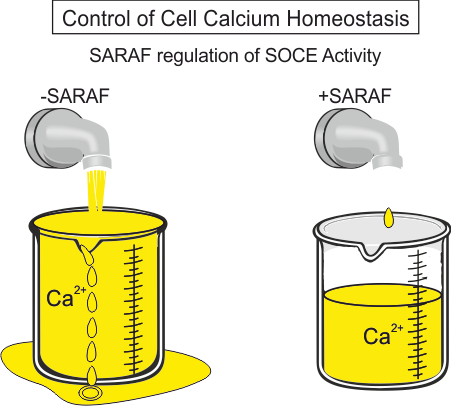 SARAF is an ER-resident protein highly conserved in all vertebrates, with exceptionally high transcript levels in the immune and neuronal tissues of mammals. During SOCE, as the concentration of Ca2+ inside the ER approaches its upper set point, SARAF associates with STIM and slowly inactivates the store-operated calcium currents. We are currently exploring the interactions between SARAF and the other members of the SOCE machinery to gain structural and mechanistic insights into its function. Using conditional knock-out mice with high-end imaging and electrophysiological techniques, we aim to delineate the physiological roles of SARAF and its significance in behaving animals.
SARAF is an ER-resident protein highly conserved in all vertebrates, with exceptionally high transcript levels in the immune and neuronal tissues of mammals. During SOCE, as the concentration of Ca2+ inside the ER approaches its upper set point, SARAF associates with STIM and slowly inactivates the store-operated calcium currents. We are currently exploring the interactions between SARAF and the other members of the SOCE machinery to gain structural and mechanistic insights into its function. Using conditional knock-out mice with high-end imaging and electrophysiological techniques, we aim to delineate the physiological roles of SARAF and its significance in behaving animals.