Ion channels are elementary excitable units integrated in the cell membranes of all cells. Their physiological roles are diverse from being responsible for the generation and propagation of nerve impulses, synaptic transmission, muscle contraction salt balance and hormone release. Thus, due to their diverse physiological role, they have been targeted pharmacologically, and many drugs have been developed, such as local and general anesthetics, muscle relaxants, cardiac anti-arrhythmic and oral hypoglycemics. Ion channels have also been found to be involved in many genetic diseases such as cystic fibrosis, cardiac arrhythmia, Liddle syndrome (hypertension) and ataxia. Thus, understanding structural and functional aspects of ion channels is of great importance.
One subset of K+ selective channels, the G protein coupled inwardly rectifying K+ channels (GIRK), are the main focus of the laboratory. These channels are involved in many physiological responses that include, regulation of heart beat rate, nociceptive pain, hormone secretion and control of seizures. Neurotransmitters such as dopamine, serotonin, adenosine and GABA exert their inhibitory actions, in part, by activating GIRK channels at the post synaptic membrane. These channels permit K+ ion flux at membrane potentials near the cell’s resting potential, thereby decreasing membrane excitability. GIRK channels, which are activated via G protein-coupled neurotransmitter receptors (GPCRs) are found in neurons, heart and endocrine tissues. In the central nervous system, for example in the hippocampus, GIRK channels were found to increase K+ conductance at the postsynaptic, but not at the presynaptic cleft, to mediate inhibitory neurotransmission (Fig 1).
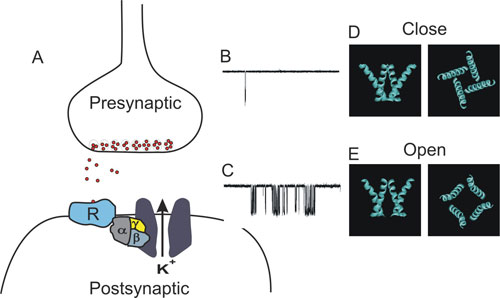
Fig. 1: Following receptor stimulation Gbg is free to gate the channel, A. Gating of GIRK single channel is mainly characterized by an increased channel bursting, B vs. C. A model depicting the second transmembrane domain rearrangement during channel gating, D.