A dire lack of potent therapies for mental illness is fueling the search for novel non-invasive therapeutic methodologies. Neuromodulation is an effective option, but none of the existing non-invasive modalities can selectively target small areas deep in the brain. Ultrasound, on the other hand, can be targeted deep into the brain with high spatial resolution.
Ultrasonic neuromodulation was first described in the early 20th century. However, large-scale attention only came to it recently when it was shown to activate neurons at intensities and frequencies that could safely be applied non-invasively through the human skull. Since then, multiple studies have demonstrated stimulation in animals and humans, but the mechanism of action has remained elusive.
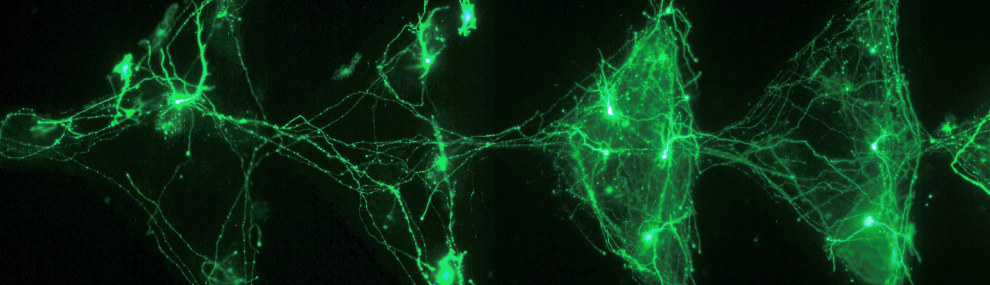
Our approach is to use neuronal cultures to investigate the mechanisms of ultrasonic neurostimulation at the single-cell level. We developed a novel experimental system whose architecture enables the application of ultrasound with minimal reflections to the cultures. We pharmacologically block synaptic transmission, eliminating network effects, enabling examination of single-cell level processes. Optical methods are used to image the activity and integrity of many hundreds of individual neurons during ultrasonic stimulation. Using pharmacological interventions, we can simultaneously disrupt specific cellular processes, revealing their role in the mechanism.
Our results detract from theories implicating cavitation, heating, membrane poration, pre-synaptic release, or gradual effects. They implicate a post-synaptic mechanism upstream of the action potential, and narrow down the list of possible targets involved. Surprisingly, we also show that even extremely short (4μs) single pulses are effective.
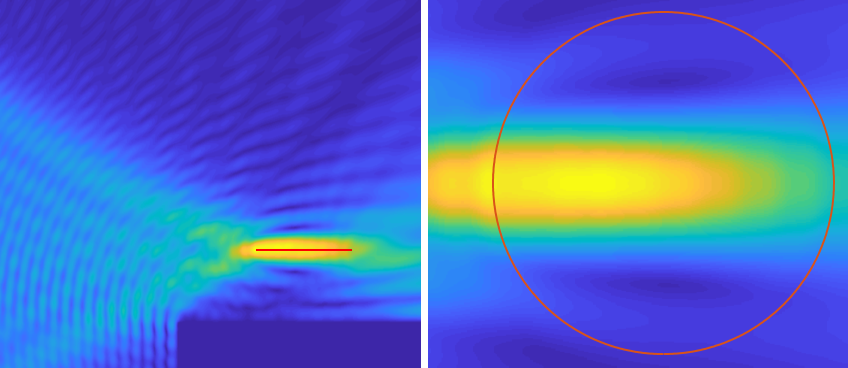
Mechanistic insights into ultrasonic neurostimulation of disconnected neurons using single short pulses
Weinreb E. and Moses E. (2022) Brain Stimulation. 15, 3, p. 769-779
Article, Supplementary