Axon-glia Communication
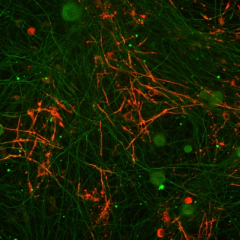
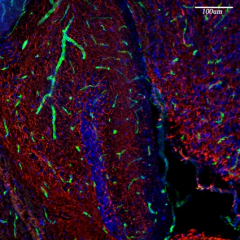
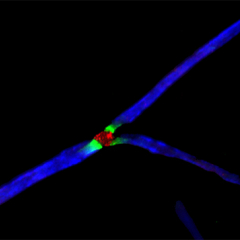
The formation and maintenance of myelinated nerve fibers requires the reciprocal communication between Schwann cells or oligodendrocytes with their underlying axons. During development, these glial cells receive specific axonal signals that control their proliferation, survival, migration and differentiation. One of the main questions we are dealing with in the lab is what are the axonal signals that enable Schwann cells and oligodendrocytes to ensheath the axons they contact and to myelinate them? We have used several molecular approaches, including a signal-sequence trap screen and a genetic cell ablation coupled with gene expression strategy to identify Schwann cells and oligodendrocytes cell surface proteins that may play a key role in axon-glia interaction and myelination. These approaches resulted in the identification of several novel cell recognition molecules (Opalin and Cadm4) and receptors (Gpr37), which we are currently studying using both in vitro myelination systems and various mouse models. We are also employing unique screening methodologies we developed to identify additional axoglial signaling components.